
Ya-Nan Ma, Teng-Long Liu, Wei-Hong Zhang, Cheng-Hang He, and Dong-Xu Xue*. Angew. Chem. Int. Ed. 2025, ASAP. DOI: https://doi.org/10.1002/anie.202513208
The pursuit of advanced adsorbents with exceptional gas adsorption and separation capability represents a highly promising yet challenging research frontier. Several quinary multicomponent metal-organic frameworks (MOFs) have been documented in the literature, however, the construction of quinary MOFs through the synergistic integration of two distinct organic ligands and three different metal clusters remains scarce.
Herein, solvothermal reaction of Zn(OAc)2·2H2O with adenine and 1,2,4-benzenetricarboxylic anhydride afforded a novel MOF of Quin-Zn-Ad-BTC. SCXRD reveals that Quin-Zn-Ad-BTC incorporates three distinct zinc-based structural units of a mononuclear [ZnN2(O2C─)2], a dinuclear [Zn2(Ad)3(O2C─)2], and a hexanuclear [Zn6N3(μ-H2O)3(Ad)3(O2C─)6], respectively. It features three interconnected cage-like cavities replete with uncoordinated carboxylate oxygen atoms and Watson─Crick sites.
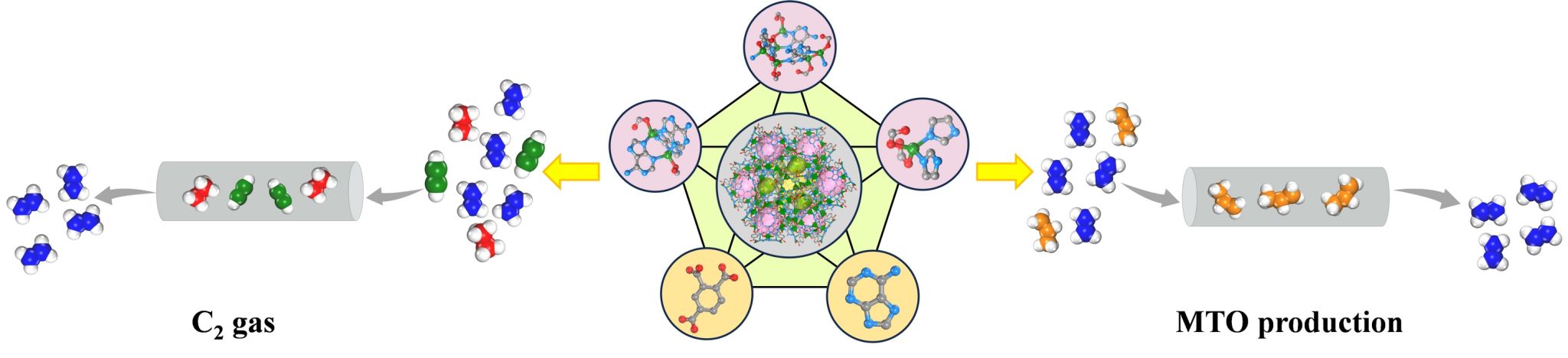
The MOF was successfully activated through multiple methanol exchanges followed by vacuum heating. It exhibits a pore volume exceeding 0.50 cm3 g-1 and a gravimetric surface area surpassing 1220 m2 g-1. At 298 K and 1 bar, its adsorption capacities for C2H2 and C2H6 consistently surpass that of C2H4. Quin-Zn-Ad-BTC demonstrates low isosteric heats of adsorption (Qst) for all three gases, indicating excellent regenerability. Ideal Adsorbed Solution Theory (IAST) calculations reveal selectivity values of 2.05 for 50/50 C2H2/C2H4 and 1.26 for 10/90 C2H6/C2H4 mixtures. Breakthrough experiments further confirm its unique capability for one-step ethylene purification from ternary C2 hydrocarbon mixtures. Additionally, C2H4 and C3H6 adsorption isotherms were measured at 298 K and 1 bar, complemented by Qst analysis and IAST predictions. Simulated breakthrough experiments with methanol-to-olefins (MTO) products demonstrate the material’s potential for separating MTO effluent streams.
In this study, we have successfully synthesized a new quinary MOF of Quin-Zn-Ad-BTC through a one-pot solvothermal reaction. Due to its distinctive structural features, the material demonstrates outstanding ethylene purification capability, enabling highly efficient one-step ethylene separation from complex C2 mixtures and MTO products under ambient pressure conditions. These findings not only validate the feasibility of quinary MOFs for advanced applications in gas adsorption and light hydrocarbon separation, but also establish a new paradigm for assembling complex multicomponent MOFs.
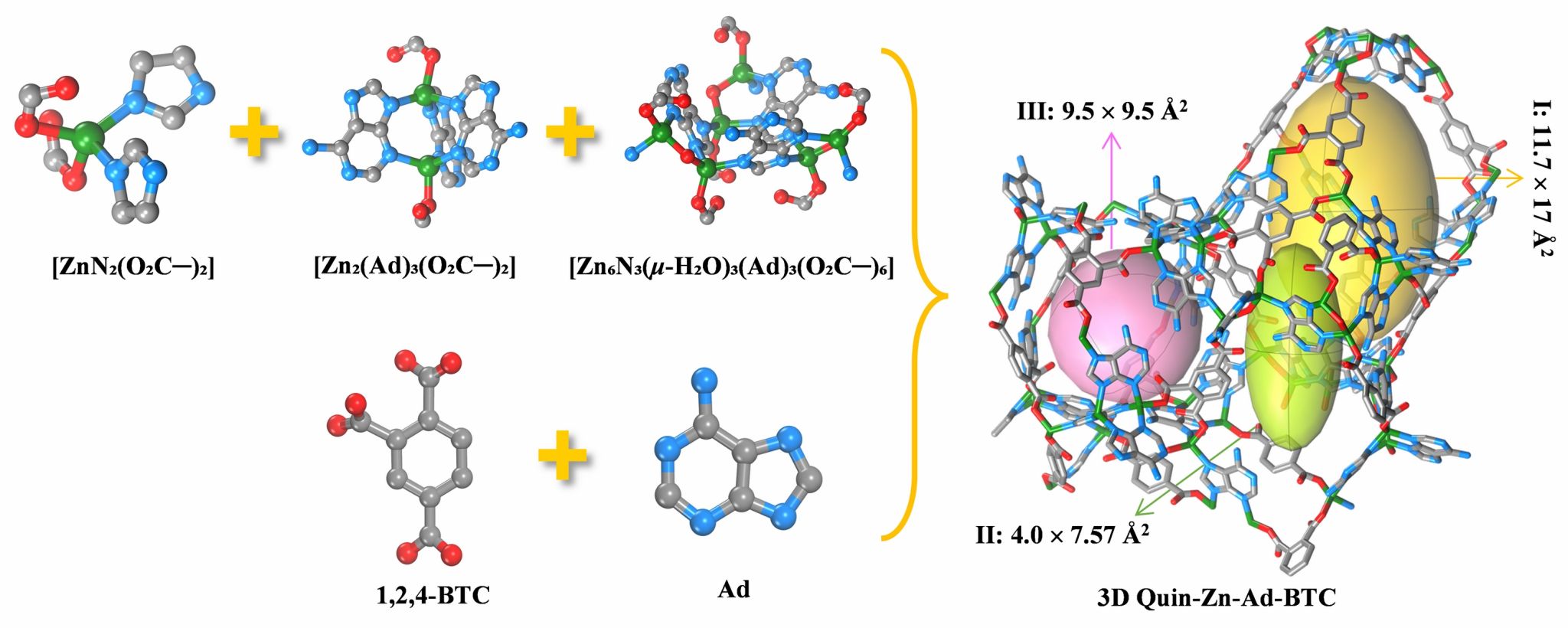
Figure 1. Schematic representation showing the assembly of Quin-Zn-Ad-BTC.
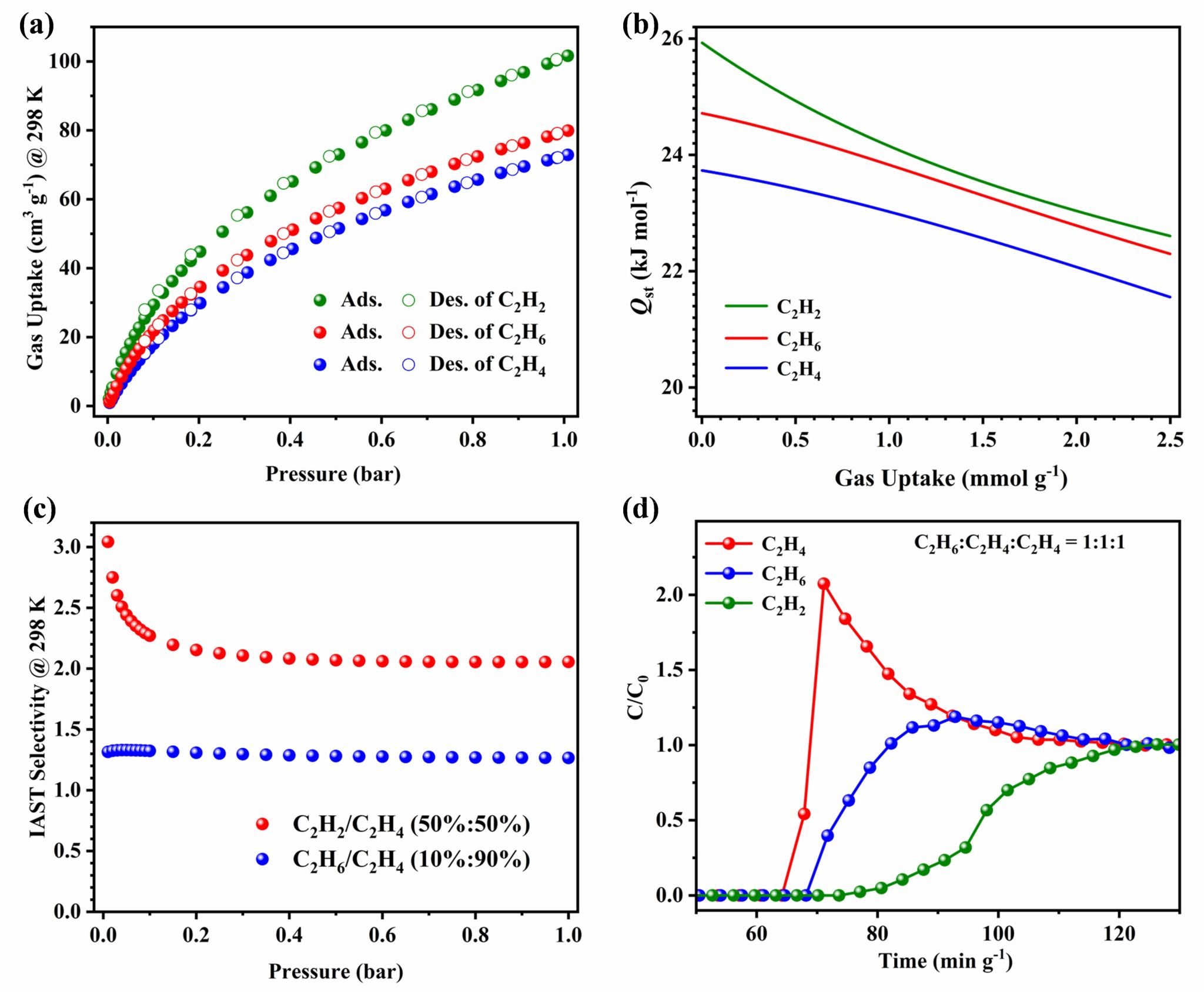
Figure 2. (a) C2H2, C2H4 and C2H6 sorption isotherms at 298 K for Quin-Zn-Ad-BTC. (b) Qst of C2H2, C2H4, and C2H6 gases. (c) IAST selectivity for 10%/90% (v/v) of C2H6/C2H4 and 50%/50% (v/v) of C2H2/C2H4 mixtures. (d) Experimental column breakthrough curves for C2H2/C2H4 /C2H6 (1/1/1, v/v/v) mixtures under a flow of 1 mL min-1.
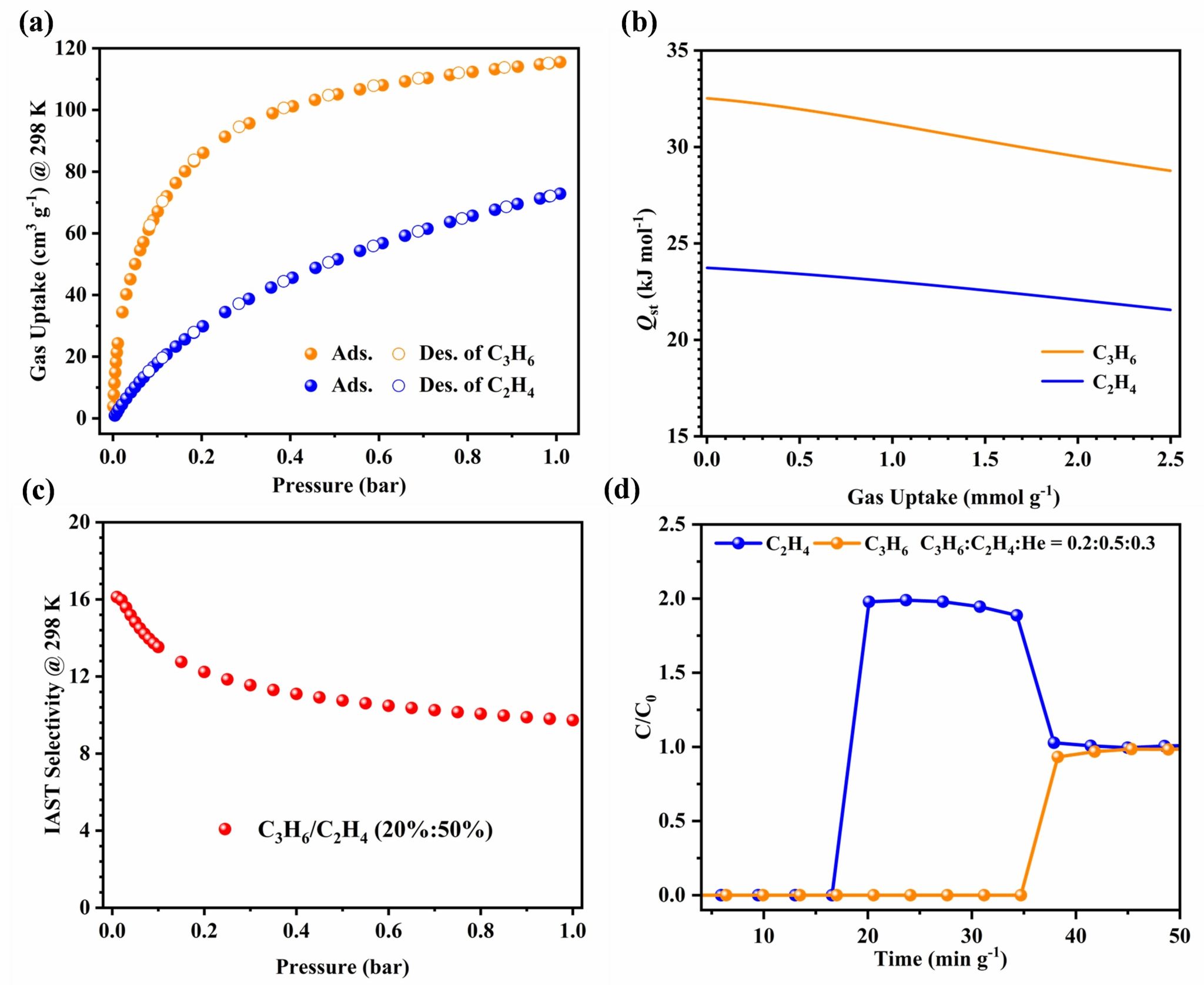
Figure 3. (a) C2H4 and C3H6 sorption isotherms at 298 K for Quin-Zn-Ad-BTC. (b) Qst of C2H4, and C3H6 gases. (c) IAST selectivity for (20%/50%, v/v) of C3H6/C2H4 mixtures. (d) Experimental column breakthrough curves for C3H6/C2H4/He (0.2/0.5/0.3, v/v/v) mixtures under a flow of 4 mL min-1.
First Author: Ma Yanan, doctoral candidate, Shaanxi Normal University
Correspondence Author: Prof. Xue Dongxu, Shaanxi Normal University
Full Text Link: https://doi.org/10.1002/anie.202513208
 Latest Updates
Latest Updates






