Baihui Wang, Yimin Bai, Jiahui Peng, Miaomiao Zhang, Weiting Zhang, Hongtao Bian*, and Yu Fang. Chin. J. Chem. Phys., 2024, 37, 398-410.
Background
The structure of protein and peptide at interfaces plays a crucial role in various biological processes and technological advancements. Understanding these structures is critical for diagnosing diseases, drug delivery and developing biomaterials. However, the complexity of these systems and limitations in analytical tools have hindered the in-depth exploration. Despite significant efforts in determining protein structures using advanced techniques like X-ray crystallography and cryo-electron microscopy, the understanding of surface-bound protein structures in real conditions remains relatively limited, posing a current challenge in this field. Vibrational sum frequency generation (SFG) spectroscopy has been developed as a versatile method for elucidating molecular structures of proteins across interfaces.
Overview
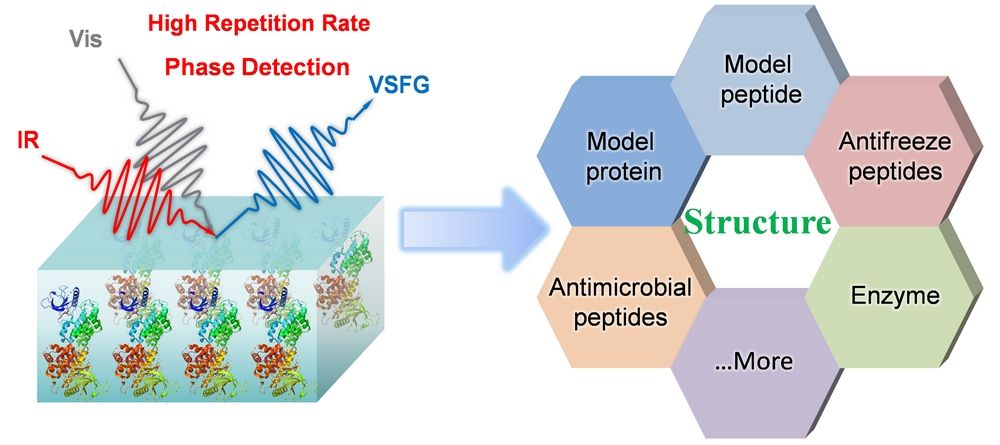
This review is intended to introduce the basic principle of SFG spectroscopy, discusses its current advancements in phase measurement, SFG is an interface-selective spectroscopy, and its signal originates from the second-order nonlinear polarizability. In a typical SFG setup, visible light and IR light are focused on the surface of the sample, overlapping in both time and space and then generate sum frequency signal. After decades of development, the techniques and methods of SFG are updated and improved. Wang’s group developed internal heterodyne phase-resolved (IHPR) SFG to obtain phase information, the same quartz crystal serves both as the SFG phase standard and the reference. By introducing a pair of ultrafast mid-IR pulses to induce a vibrational transition, the dynamic information related with the energy transfer at interfaces can be obtained. Ye group used this method to study the ultrafast dynamics of a variety of biomolecules at interfaces. Recently, Wang and coworkers developed a cost-effective version of sub-wavenumber SFG spectrometer. This innovation presents a more affordable and accessible approach to conducting powerful high resolution SFG studies, ideal for diverse applications in examining molecular surfaces and interfaces’ structures and dynamics.
Research on the study of proteins at interface using SFG initially focused on investigating the physical interactions between protein side chains and polymer surfaces. Numerous studies have centered on the analysis of bovine serum albumin, lysozyme, and fibrinogen. However, the intricate nature and repetitiveness of amino acid side chains pose challenges in interpreting protein SFG spectra in terms of main chain folding or side chain structure. Consequently, many experiments employ synthetic model peptides to enable a detailed examination of protein-surface interactions at the molecular level. The exploration of model peptides has laid the groundwork for strategies in determining protein orientation, particularly through the analysis of side chain methyl groups and amides. This concise review aims to establish a foundation for future studies and applications exploring different types of peptides and proteins at interfaces using SFG.
Opportunities and Challenges
Although SFG spectroscopy holds promise for addressing various issues, it still encounters several challenges. These include: (i) Previous applications have primarily focused on elucidating the secondary structure of single-component systems, with limited exploration of tertiary and higher-order structures of proteins. (ii) The complexity of the anticipated vibrational spectrum remains challenging to interpret, particularly regarding phenomena such as the coupling between the O-H extension of water and the N-H extension of a protein polypeptide. (iii) A common challenge across laser spectroscopy methods is achieving system stability, ease of operation, and user-friendly interfaces for integration with other techniques, along with the preparation of high-quality biological samples that truly reflect biological relevance, often relying on model systems at present.
First Authors: Doctoral candidate Wang Baihui, master’s candidate Bai Yimin, Shaanxi Normal University
Correspondence Author: Prof. Bian Hongtao, Shaanxi Normal University
Full Text Link: https://cjcp.ustc.edu.cn/hxwlxb/en/article/doi/10.1063/1674-0068/cjcp2312146