
Zhen Yan, Rongrong Zhang, Min Qiao, Miao Ma, Taihong Liu,* Liping Ding,* and Yu Fang. Anal. Chem. 2024, 96, 13801−13810 DOI: 10.1021/acs.analchem.4c01287
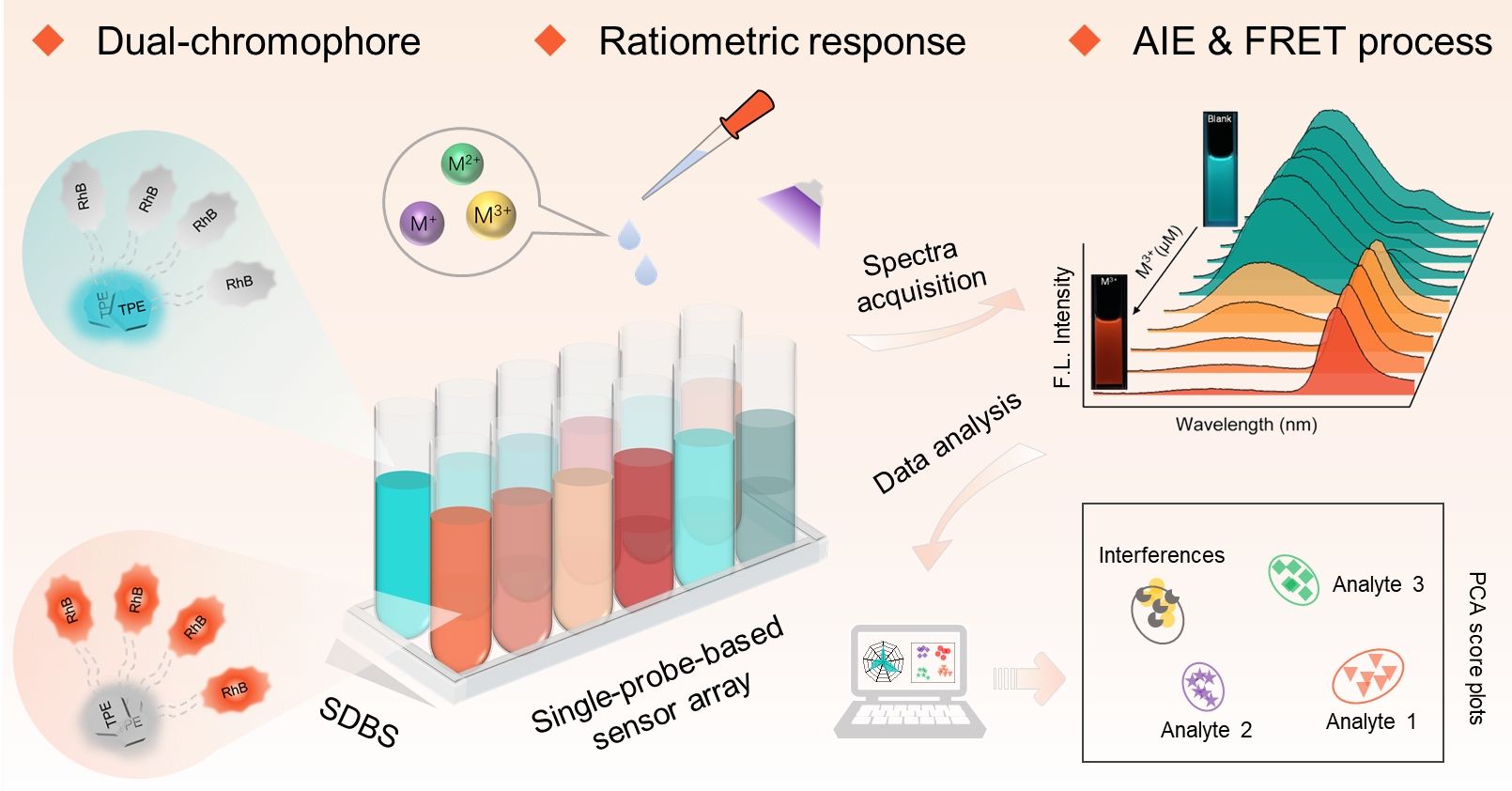
Trace determination and toxicity evaluation of heavy metal ions have been extensively considered in the fields of water quality monitoring, medical diagnosis, food safety control, etc. Their maximum contaminant levels under specific conditions are strictly limited following the World Health Organization (WHO) guidelines. Among various trivalent metal ions (M3+), Fe3+, Al3+, and Cr3+ act either significantly in many physiological and pathological processes or exist widely in our lives. On the other hand, abnormal concentration levels of M3+ might hinder their natural biological activities in physiological processes and cause severe health hazards. Consequently, tracking the M3+ ions in the environment and biological systems is of great significance. fluorescent sensors have evoked continuous attention because of their merits of easy operation, high sensitivity, real-time detection, etc. Numerous studies based on fluorescence changes at a specific wavelength have been reported, but selectivity and discrimination concerns may limit their practical utilizations. With the advantages of multiple emission bands, ratiometric characteristics, and cross-reactive response, single-component fluorescence sensor arrays and dual-chromophore probes have emerged as attractive tools for favorably solving the aforementioned problems to a certain extent in recent years.
Rhodamine (RhB) units are known to possess marked transformations from the colorless closed-ring form of the spirolactam structure to the strong orange ring-opened structure, and are widely used as sensing platforms for a variety of analytes due to its stimulus-response switching properties. Tetraphenylethene (TPE) analogues are found to be nonfluorescent or weakly emissive in molecular states but emit intensely in aggregate states due to the restriction of intramolecular rotation (RIR). The photophysical properties of TPE as natural self-assembling building blocks can be easily modulated by molecular aggregation behavior. Therefore, a multiple-signal sensing platform could be built through RhB-TPE as a dual chromophore provides AIE properties accompanied by the analyte switching response of RhB emission and further integrated FRET process (Figure 1). In this contribution, a dual-chromophore probe (RhB-TPE) composed of conjugated TPE and RhB units linked via a long alkyl-oxygen chain was developed. The probe RhB-TPE intrinsically aggregated into sphere-shape in ACN/H2O and exhibited special AIE properties. Single-probe-based sensor array was further constructed by co-assembling with the anionic surfactant SDBS at two different concentrations. Following the surfactant modulation strategy, the two-element array showed cross-reactive and discriminative responses to three M3+ ions (Fe3+, Al3+, and Cr3+) in aqueous media. The cross-reactive sensing mechanism was attributed to M3+ influences on an efficient FRET process from TPE to open-ring form RhB. With the coexistence of specific Al3+, the obtained ensemble array was further applied to discriminate different types of brand water (Figure 2).
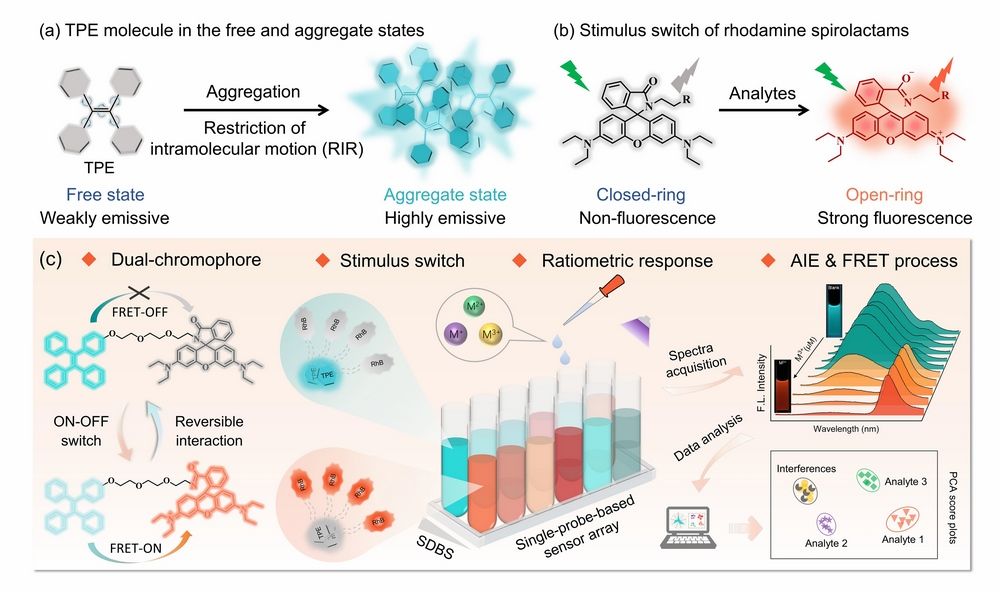
Figure 1 (a) Non-fluorescent TPE in free states but strongly emissive in aggregates due to RIR. (b) Stimulus switch of rhodamine spirolactam unit from the non-fluorescent closed-ring form to fluorescent open-ring form. (c) Representative scheme of the single-probe-based two-element sensor array for discriminating M3+ analytes.
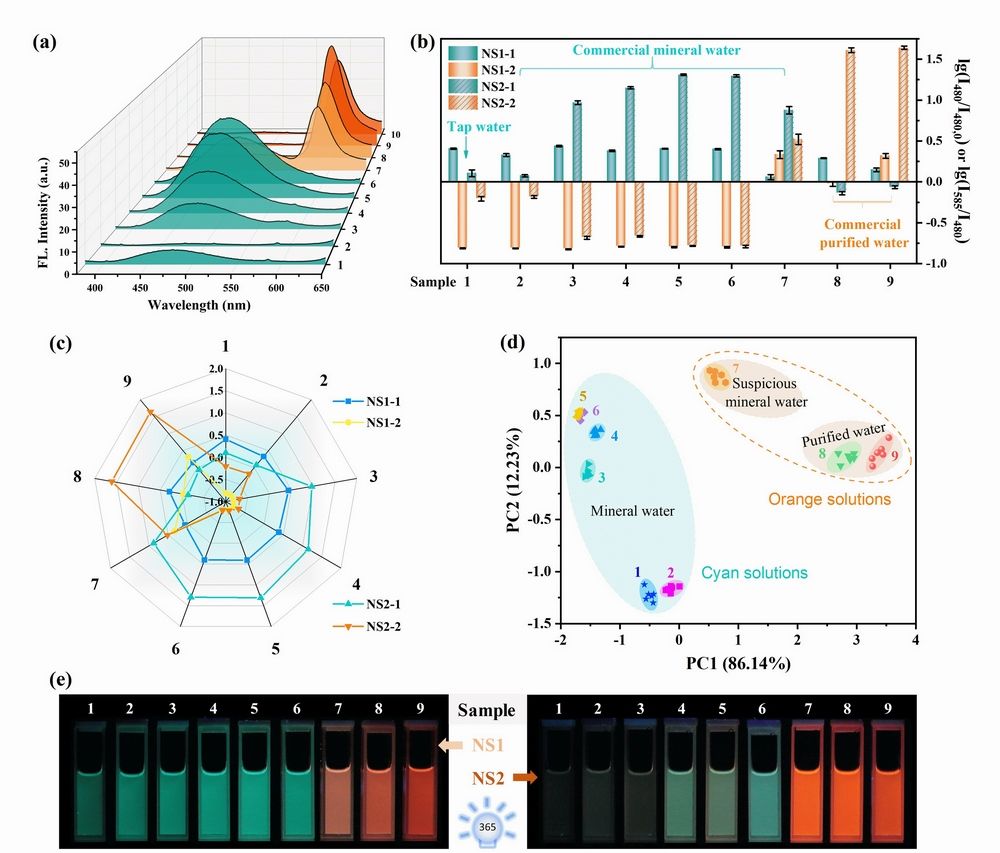
Figure 2 (a) Fluorescence emission spectra of sensor element NS2 in different types of water. Fluorescence responses of Sensor Array 2 to nine different drinking water samples (1-9) by collecting fluorescence variations at 480 and 585 nm: (b) four-signal recognition patterns, (c) radar plots, and (d) PCA plots. NS1-1 and NS1-2 represent lg(I480/I480,0) and lg(I585/I480) acquired from sensor element NS1, respectively. NS2-1 and NS2-2 acquired from sensor element NS2. (e) Photographs of Sensor Array 2 in different water samples (1-9). Left: NS1; Right: NS2.
First Author: Yan Zhen, doctoral candidate, Shaanxi Normal University
Corresponding Authors: Prof. Ding Liping, Assoc. Prof. Liu Taihong, Shaanxi Normal University
Full Text Link: https://doi.org/10.1021/acs.analchem.4c01287