Rongrong Huang,# Qinglong Qiao,# Deborah Seah, Tianruo Shen, Xia Wu, Fabio de Moliner, Chao Wang, Nannan Ding, Weijie Chi, Huaming Sun, Marc Vendrell,* Zhaochao Xu,* Yu Fang,* and Xiaogang Liu*. J. Am. Chem. Soc. 2025, 147, 5258-5268. DOI: 10.1021/jacs.4c16087

Organic fluorophores are widely used in life sciences and biomedical research, including applications in disease diagnosis, protein quantification, and studies of cellular dynamics. However, traditional fluorophores often suffer from large molecular sizes and short emission wavelengths, limiting their utility in deep-tissue imaging and high-resolution microscopy. Developing fluorophores that combine compact molecular dimensions with near-infrared (NIR) emission has remained a significant scientific challenge.
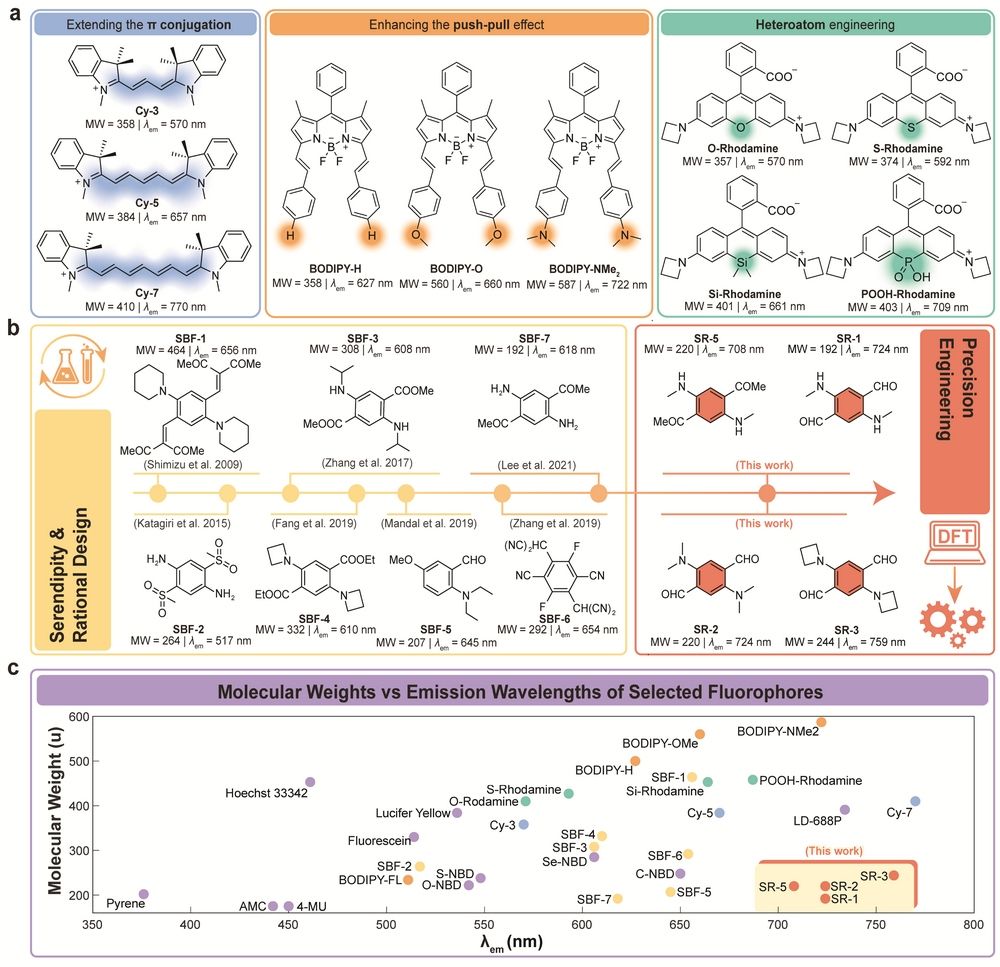
Figure 1. Schematic Representation for Developing Red/NIR Fluorophores and Single-Benzene Fluorophores.
In this work, we established an iterative design methodology through quantum chemical calculations and structure-property relationship analysis, enabling precise structural engineering of single-benzene-based NIR fluorophores. By systematically introducing electron donors (D) and acceptors (A), optimizing push-pull electronic effects, and managing steric hindrance, we determined optimal substituent positions, quantities, and types. This approach yielded fluorophores with ultra-low molecular weights and extended emission wavelengths. For example, SR-1 exhibits a molecular weight of 192 g/mol and an emission peak at 724 nm, while SR-3 achieves an emission wavelength of 759 nm.
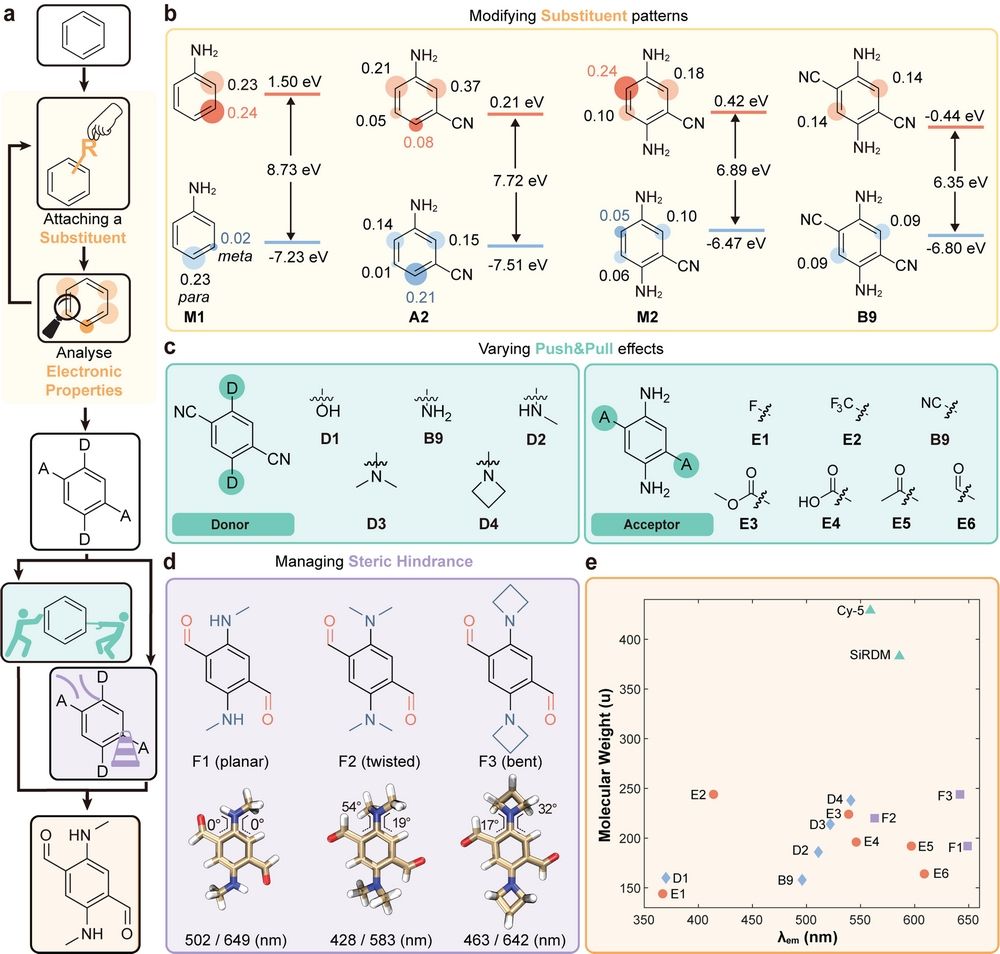
Figure 2. Iterative precision molecular engineering of small and red/NIR fluorophores.
These novel fluorophores demonstrate unique environmental sensitivity: they are nearly non-emissive in aqueous solutions but highly fluorescent in lipid environments. This property enables wash-free live-cell imaging, simplifying experimental workflows while maintaining high signal-to-noise ratios. Their photostability and selectivity further facilitate super-resolution imaging, allowing prolonged tracking of lipid droplet dynamics. By conjugating the fluorophores with D-alanine, the team developed ASR-5, a fluorescent unnatural amino acid that successfully labels bacterial cell wall biosynthesis. This innovation provides a powerful tool for bacterial research and antibiotic development. Additionally, the compact molecular size of these dyes permits penetration into microcavities (e.g., molecular sieves), opening new possibilities for materials science and 3D imaging.

Figure 3. Wash-free imaging of lipid droplets (LDs) in live cells using SR fluorophores.
This study not only resolves the long-standing contradiction between small molecular size and long emission wavelengths in fluorophore design but also expands the frontiers of bioimaging through precision molecular engineering. With further optimization and application exploration, these compact NIR fluorophores hold promise for advancing scientific research and clinical diagnostics. Although improvements in molar absorption coefficients and functionalization are still needed, the universal applicability of this design paradigm establishes a new framework for the rational development of functional fluorescent probes.
By overcoming the technical bottleneck of reconciling compact molecular dimensions with NIR emission, this work pioneers an innovative pathway for high-resolution bioimaging. As performance enhancements and application scenarios evolve, these ultra-compact NIR fluorophores are poised to become pivotal tools in life science research and precision clinical diagnostics. While challenges in boosting molar absorptivity and functional modifications persist, the proposed iterative design strategy—guided by quantum chemical calculations—offers a transformative paradigm for the rational design of function-oriented fluorescent dyes.
First Authors: Huang Rongrong, Singapore University of Technology and Design, Shaanxi Normal University; Qiao Qinglong, Dalian Institute of Chemical Physics, Chinese Academy of Sciences
Correspondence Authors: Liu Xiaogang, Singapore University of Technology and Design; Fang Yu, Shaanxi Normal University; Xu Zhaochao, Dalian Institute of Chemical Physics, Chinese Academy of Sciences; Marc Vendrell, The University of Edinburgh
Full Text Link: https://doi.org/10.1021/jacs.4c16087