
Yali Hou+, Yingjie Li+, Dengke Han+, Zeyuan Zhang, Jinping Zhang, Shijin Jian, Zixuan Li, Xianglong Duan*, Haonan Peng*, Yu Fang, and Mingming Zhang*. Angew. Chem. Int. Ed. 2025, e202507112. DOI: 10.1002/anie.202507112.
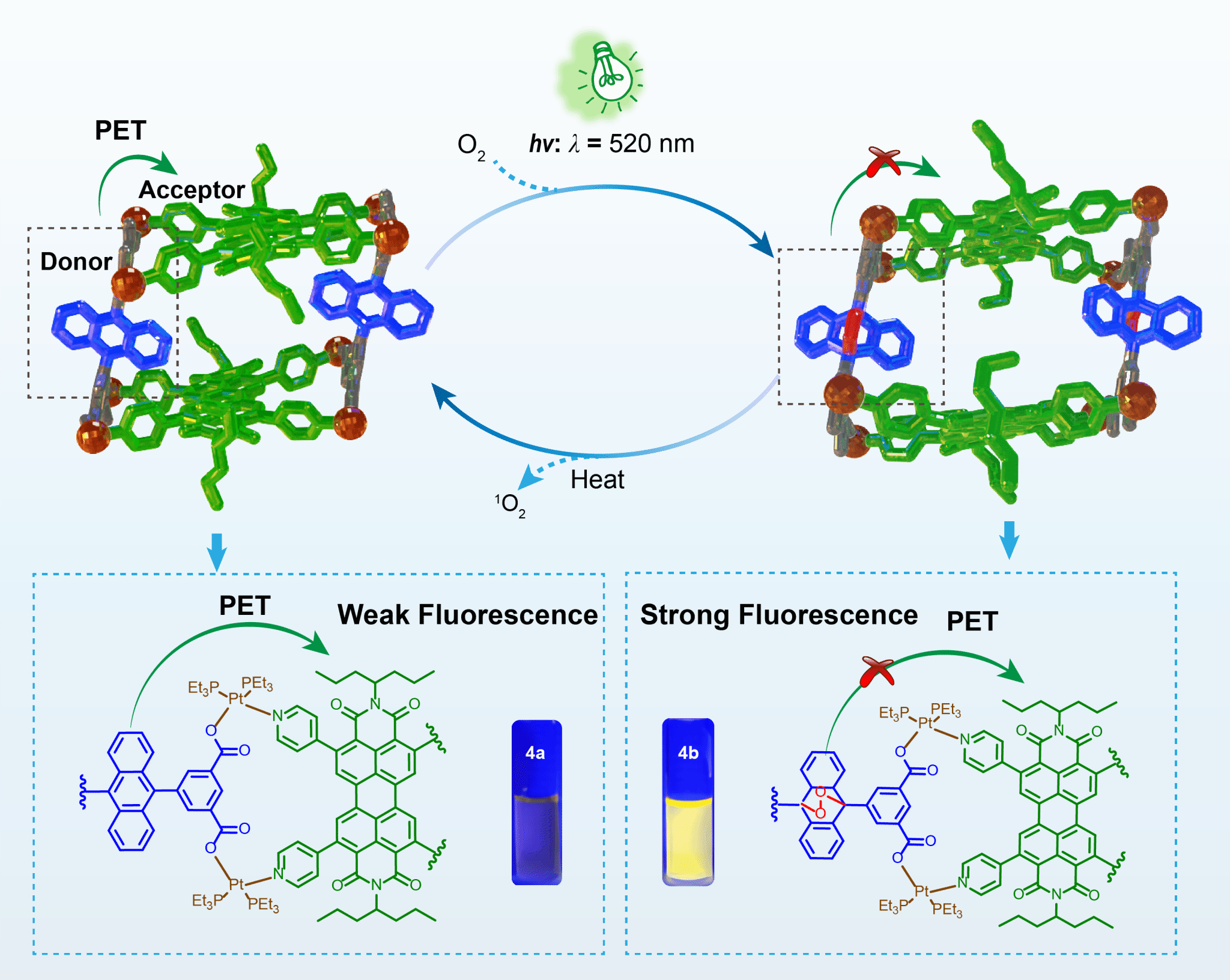
Singlet oxygen (1O2), an excited state of molecular oxygen, is widely used in photodynamic therapy, wastewater treatment, and photocatalytic oxidation due to its oxidative properties. However, its short lifetime and challenges in controlled storage/release limit practical applications. Aromatic compounds like anthracene derivatives react with 1O2 to form endoperoxides (EPOs) for reversible storage, but their instability and poor solubility hinder optimization. Additionally, external photosensitizers are often required to generate 1O2. Integrating 1O2 generation, capture, storage, and release into a single system remains critical for advancing applications.
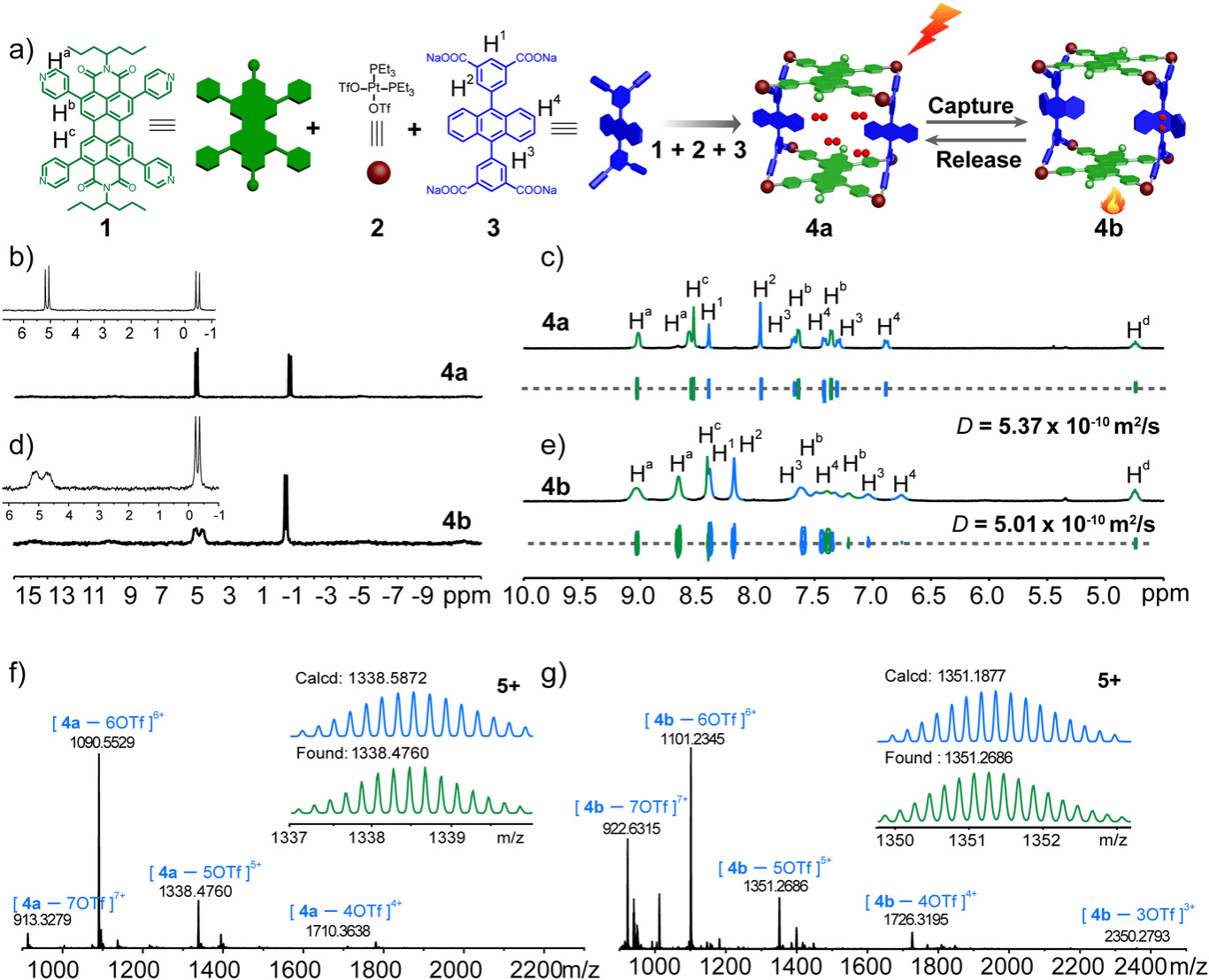
Figure 1. Self-assembly of PDI-based metallacage 4a and its peroxidation to metallacage 4b.
Coordination-driven self-assembly allows precise construction of supramolecular architectures, such as metal-organic frameworks (MOFs), which enable controlled 1O2 release. However, molecular metallacages have not yet achieved this functionality. Metallacages could combine 1O2 dynamics with optical properties for real-time monitoring, but integrating photosensitizers and EPO precursors into a single cage requires precise ligand design.
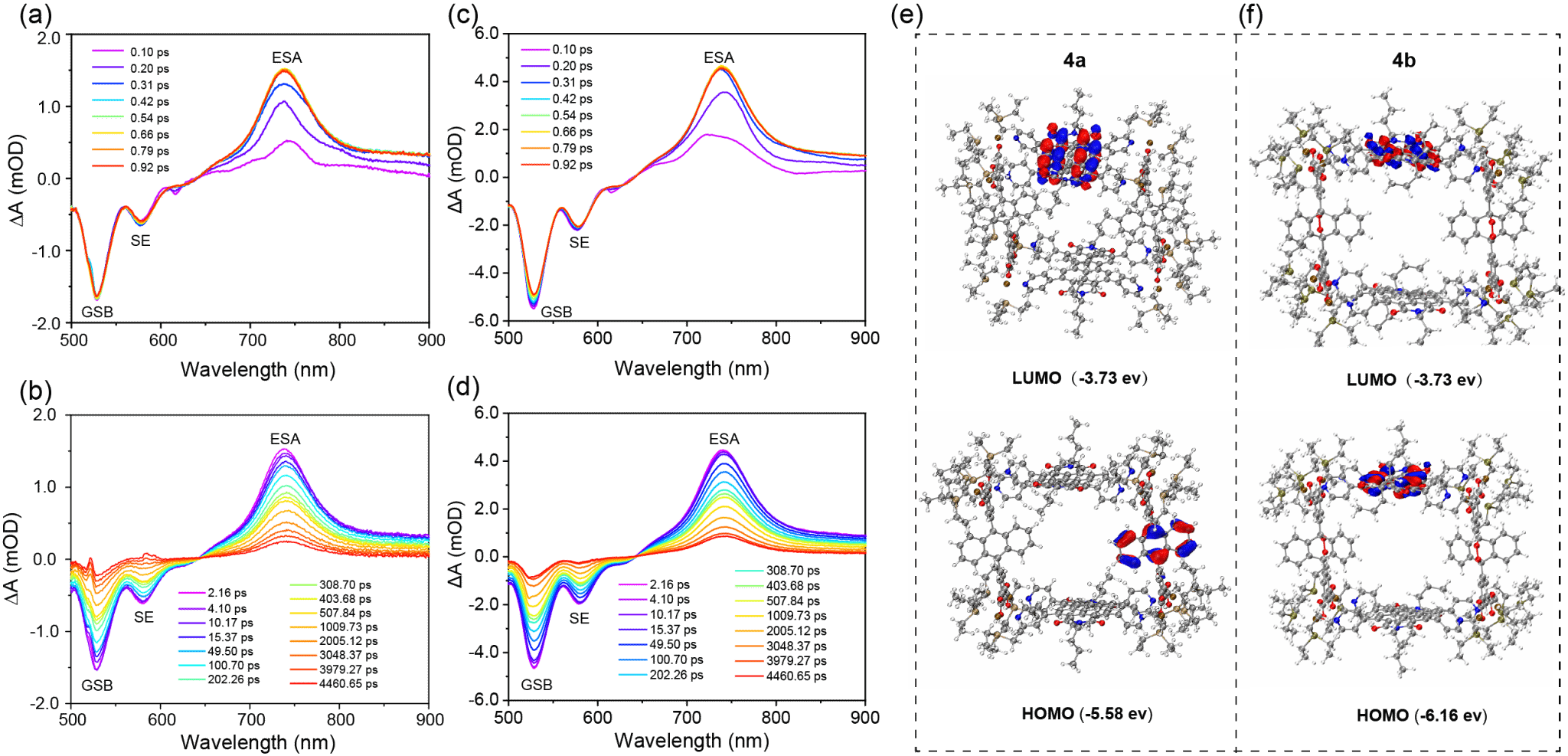
Figure 2. fs-TA spectra and diagram of the HOMO–LUMO calculated energy profiles of 4a and 4b.
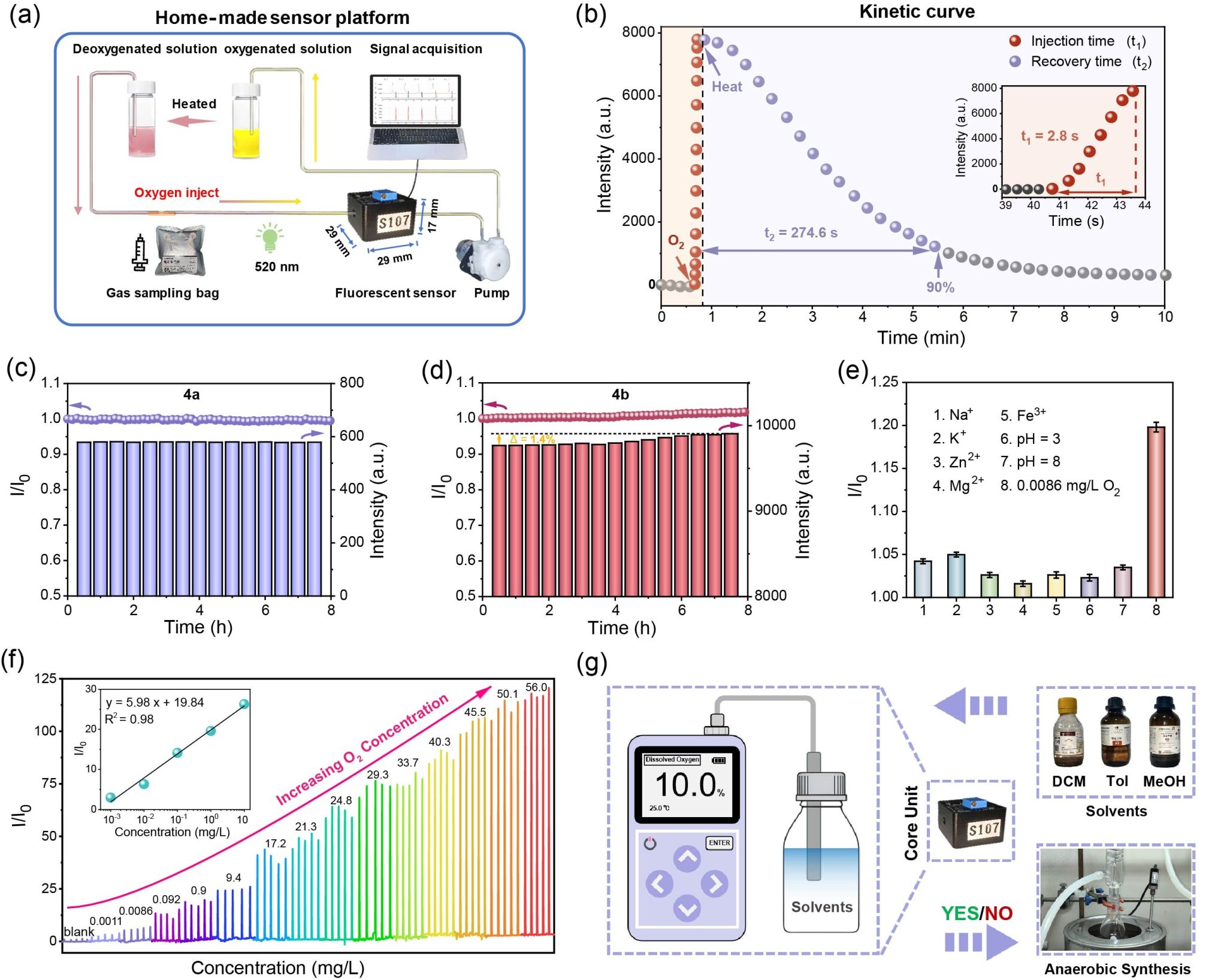
Figure 3. Sensing performance of the PDI-based sensor for trace dissolved oxygen detection.
Here, we design a box-shaped metallacage (4a) via self-assembly of tetrapyridyl perylene diimide (PDI) and tetracarboxylate anthracene. Upon irradiation, PDI generates 1O2, which reacts with anthracene pillars to form peroxidized metallacage 4b, storing 1O2. Heating releases 1O2 and regenerates 4a, confirmed by X-ray diffraction. Photoinduced electron transfer (PET) from anthracene to PDI in 4a quenches fluorescence, while peroxidation in 4b inhibits PET, restoring emission.
This reversible emission change enables the metallacage to function as a “turn-on” fluorescence sensor for dissolved oxygen, with a detection limit of 1.1 × 10−3 mg/L. This study demonstrates a metallacage that can generate, capture, store, and release 1O2 for oxygen sensing, offering insights for designing stimuli-responsive smart materials.
First Authors: Hou Yali, Postdoctoral researcher, Xi’an Jiaotong University; Li Yingjie, doctoral candidate, Shaanxi Normal University; Han Dengke, master’s student, Xi’an Jiaotong University
Correspondence Authors: Prof. Peng Haonan, Shaanxi Normal University; Prof. Zhang Mingming, Xi’an Jiaotong University; Prof. Duan Xianglong, Shaanxi Provincial People’s Hospital
Full Text Link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202507112