
Qiuping Wang (#), Yalei Ma (#), Yutong Shang (#), Taihong Liu, Xiaoyan Liu, Jing Liu, Liping Ding, Rong Miao,* and Yu Fang. Angew. Chem. Int. Ed. 2025,e202514295. DOI: https://doi.org/10.1002/anie.202514295
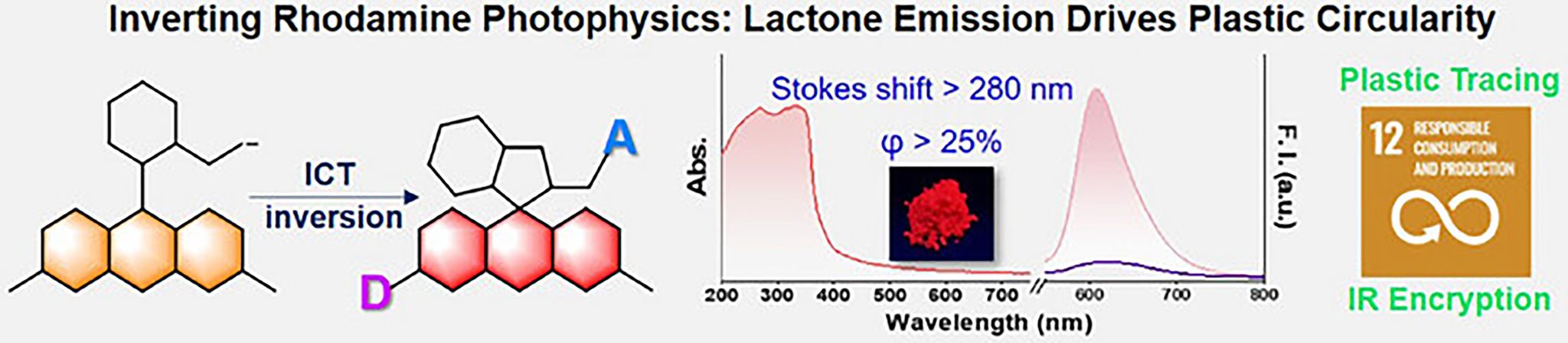
Rhodamine-based compounds play crucial roles in numerous fields such as chemical sensing, bioimaging, laser dyes, and fluorescence labeling. A key structural feature of these molecules is the dynamic equilibrium between the lactone (closed-ring form) and zwitterionic (open-ring form) states. Conventional research has predominantly focused on the zwitterionic form due to its tunable fluorescence properties and absorption band in the visible region. However, typical rhodamine derivatives often suffer from small Stokes shifts and aggregation-caused quenching (ACQ), which significantly limit their performance, particularly in solid-state applications. Therefore, developing new rhodamine systems that exhibit large Stokes shifts and efficient solid-state emission is of great importance.
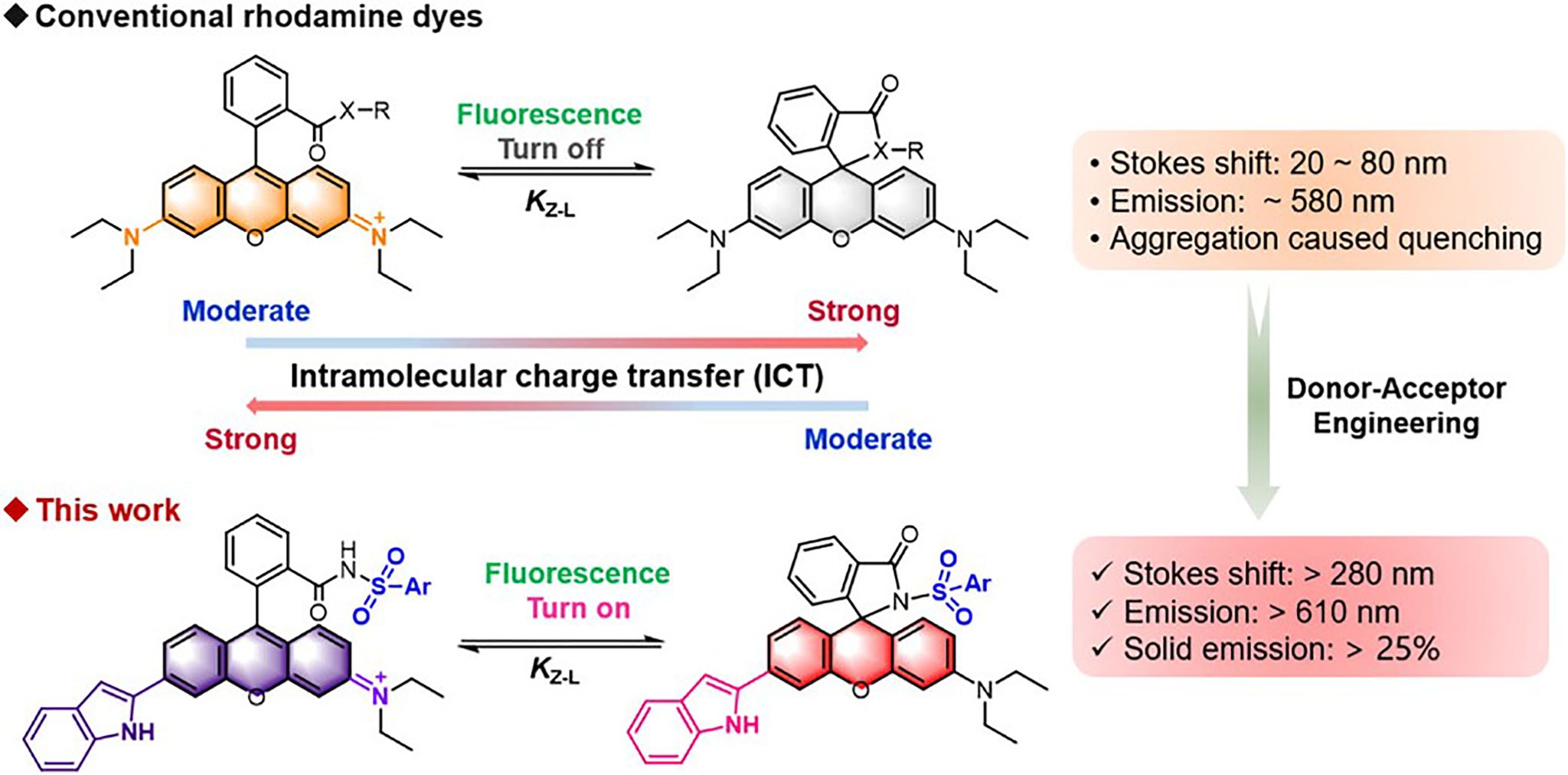
Figure 1. Comparison between conventional rhodamines and the rhodamines in this work.
This work introduces a donor–acceptor engineering strategy to activate fluorescence emission from the closed-ring form of rhodamine, achieving an unprecedented Stokes shift (>280 nm) and overcoming ACQ. Specifically, by replacing the N,N-diethyl group with an indole moiety (donor) and converting the spirolactam into a phenylsulfonamide (acceptor), we synthesized a new class of rhodamine derivatives that exhibit reversed open–closed ring fluorescence behavior compared to conventional rhodamines. Single-crystal X-ray diffraction confirmed the closed-ring configuration. Structural analysis revealed that the incorporation of indole and phenylsulfonamide results in a three-dimensional molecular geometry, which inhibits close packing and thus excitonic coupling, leading to bright fluorescence in the solid state. Some of the compounds showed solid-state absolute quantum yields exceeding 25%. Density functional theory calculations revealed that the new fluorophores exhibit intramolecular charge transfer (ICT) characteristics opposite to those of rhodamine B, with moderate ICT strength corresponding to the bright state, while strong ICT leads to fluorescence quenching.
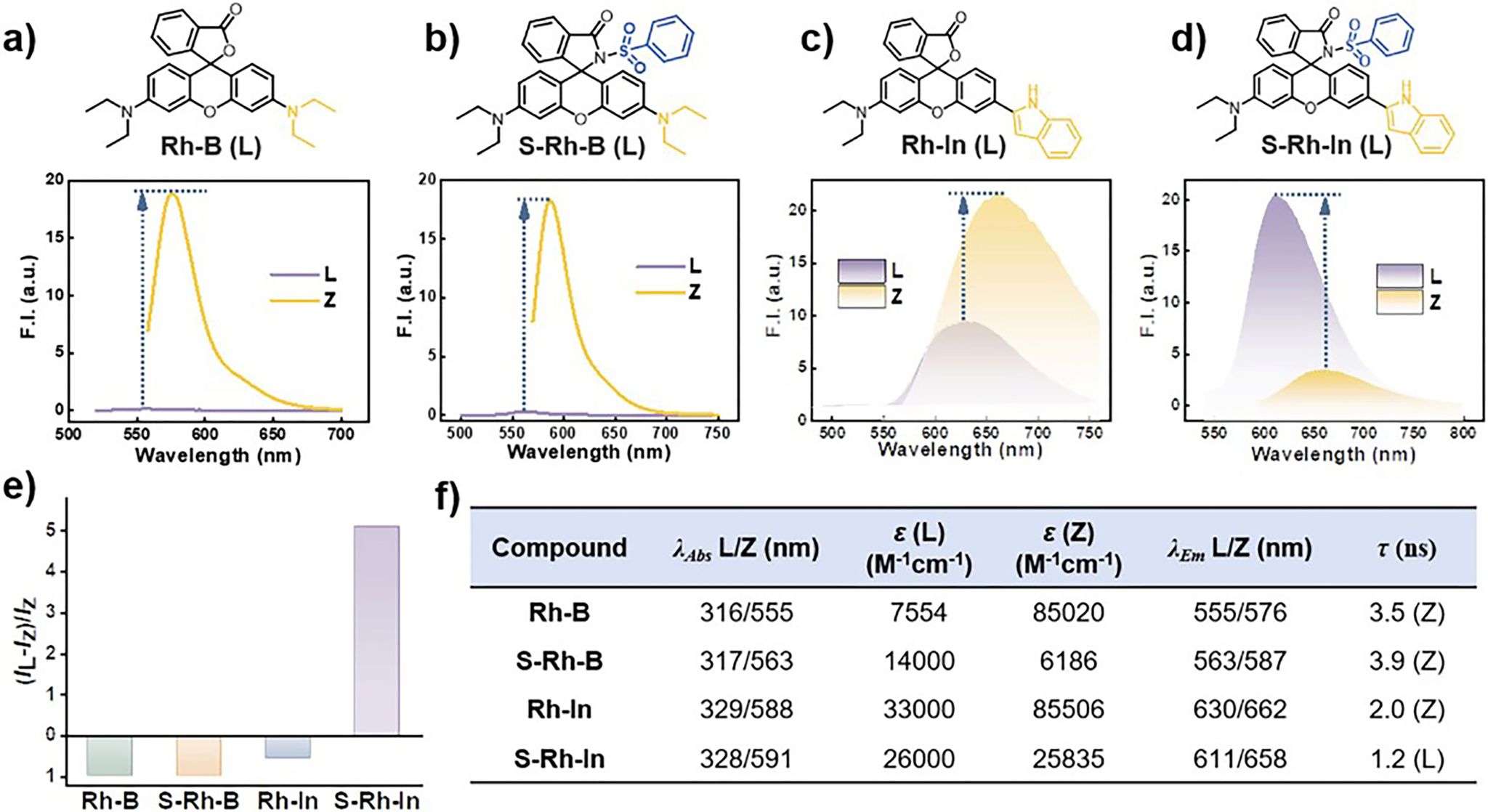
Figure 2.Rhodamine dyes with varied substitution groups. a)–d) Chemical structures and fluorescence emission of Rh-B, S-Rh-B, Rh-In, and S-Rh-In in different forms (L: lactone; Z: zwitterion). e) Fluorescence enhancement of the dyes upon the Z to L transition (IL: fluorescence intensity of L; IZ: fluorescence intensity of Z). f) Photophysical properties of Rh-B, S-Rh-B, Rh-In, and S-Rh-In in dichloromethane. Dye concentration: 5 × 10−6 M. Triethylamine (1% v/v) and trifluoroacetic acid (1% v/v) solutions were used to ensure L and Z states during the measurement.
Leveraging the unique photophysical properties of these novel rhodamine compounds, we proposed a closed-loop plastic recycling approach: using the closed-ring form for fluorescence tracking, while acid-treated (open-ring) labeled non-recyclable plastics can be repurposed as security inks. This strategy offers an innovative application of rhodamine dyes in the plastic circular economy, providing new insights into engineering rhodamine-based fluorescence and contributing to plastic sustainability.
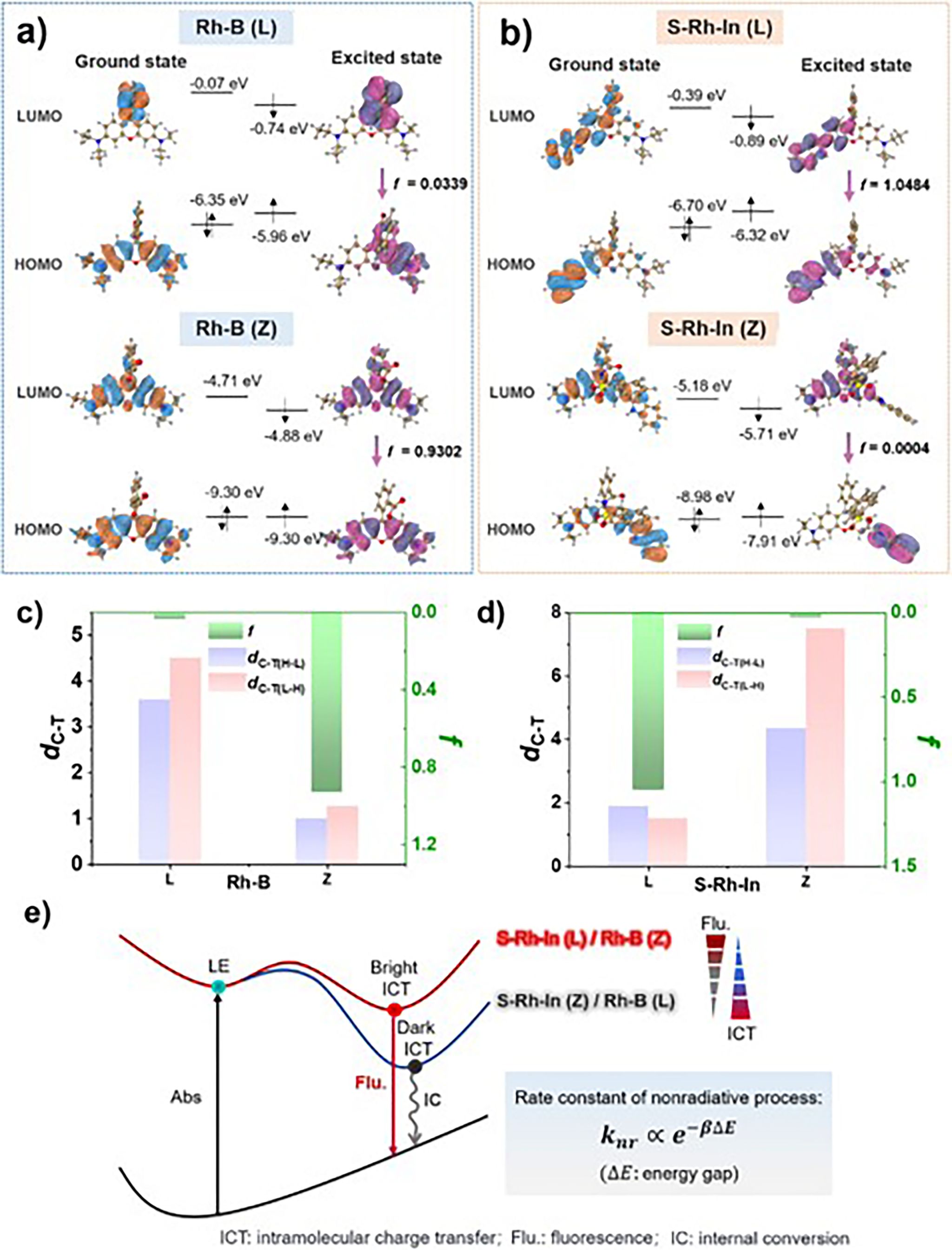
Figure 3. Frontier orbitals of Rh-B a) and S-Rh-In b) in the open form (Z) and closed form (L). Calculated C-T distance (dC-T), and oscillator strength (f)in L/Z of Rh-B (c) and S-Rh-In d). e) Proposed Jablonski diagram for Rh-B and S-Rh-In, showing the bright ICT and dark ICT states.
First Authors: Wang Qiuping, Shang Yutong, master’s students, Ma Yalei, doctoral candidate, Shaanxi Normal University
Correspondence Author: Assoc. Prof. Miao Rong, Shaanxi Normal University
Full Text Link: https://doi.org/10.1002/anie.202514295