
Wang Guan, Wenhe Jiang, Xinling Deng, Wansheng Tao, Jiaqi Tang, Yuangang Li, Jianhong Peng, Cheng-Lung Chen, Kaiqiang Liu*, Yu Fang. Angew. Chem. Int. Ed., 2023, 62, e202303506.
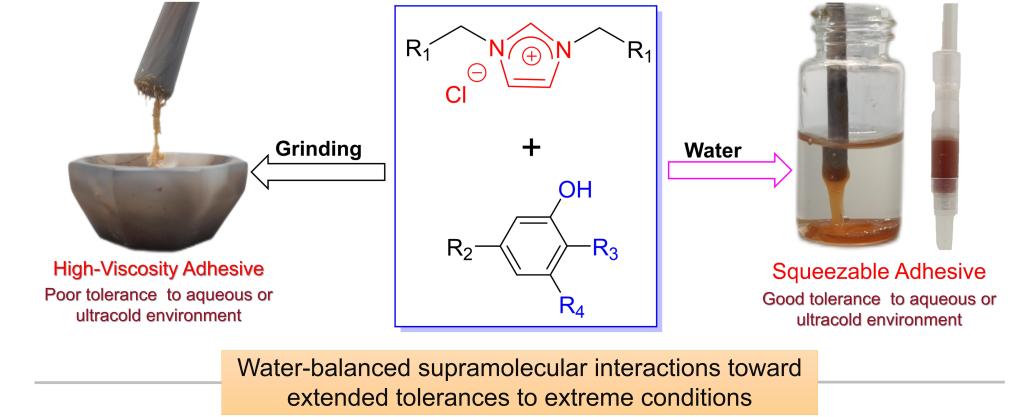
Supramolecular adhesives have attracted widespread attention due to their advantages, e.g. strong designability and dynamic reversibility of interfacial adhesion. However, it is precisely because the noncovalent interactions are more vulnerable to external influences than the covalent bond, developing supramolecular adhesives with excellent environmental tolerance is of great challenges. As is well-known, the key to determining the application performance of supramolecular adhesives for multiple substrates lies in their ability to adapt to various environments (e.g., air, water, acids, organic solvents, or liquefied gas, etc.) and clarifying at the molecular level how supramolecular interactions affect the interfacial adhesion performance of supramolecular adhesives.

Figure 1. 1H NMR titration with various additives: Cation-p interaction vs Hydrogen bonding

Figure 2. Molecular dynamics simulation: Changes of balancing supramolecular interactions with the participation of water in the adhesive system

Figure 3. Interfacial adaptability: Cases for real applications
On the basis of the research on spatially confined crystallization in supramolecular gels, we have developed a series of supramolecular adhesives derived from small molecule imidazole-based ionic liquids and polyphenolic tannins as raw materials by using the supramolecular balance regulation strategy. The resulting adhesives exhibit efficient interfacial adhesion performance under various conditions on various substrates with a bonding strength of up to 10.0 MPa. The results confirmed that the water participation extends the ultralow temperature resistance and solvent resistance of interfacial adhesion, also making the environmental adaptability of the adhesive more extensive.
Furthermore, the mechanism of the interfacial adhesion was explored through 1H NMR titration and molecular dynamics simulation, and the differences in supramolecular interactions of the system before and after water participation were clarified. The analysis revealed that the hydration of anions and cations is the key factor affecting adhesive-adhesive interactions, solvent-adhesive interactions, and adhesive-substrate interactions, etc. On this basis, a universal strategy for preparing adhesives by combining ionic liquids with phenols was further developed.
The significance of this work lies in: (1) discovering that water participation changes the interactions between adhesive-adhesive, and adhesive-substrate, which is the key to low temperature tolerance and solvent tolerance of the adhesive, and revealing that water participates in balancing supramolecular interactions at the molecular level; (2) A general method for preparing two-component supramolecular adhesives has been developed through supramolecular combination strategy. All the findings lay the foundation for the subsequent development of functional adhesives.
First Author: Guan Wang, master’s candidate, Shaanxi Normal University
Corresponding author: Prof. Liu Kaiqian, Shaanxi Normal University
Full Text Link: https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.202303506