
Tingyi Wang, Xiangquan Liu, Jinglun Yang, Jiaqi Tang, Binbin Zhai, Yan Luo, Zhongshan Liu*, and Yu Fang*. Langmuir, 2024, 40, 4489-4495.
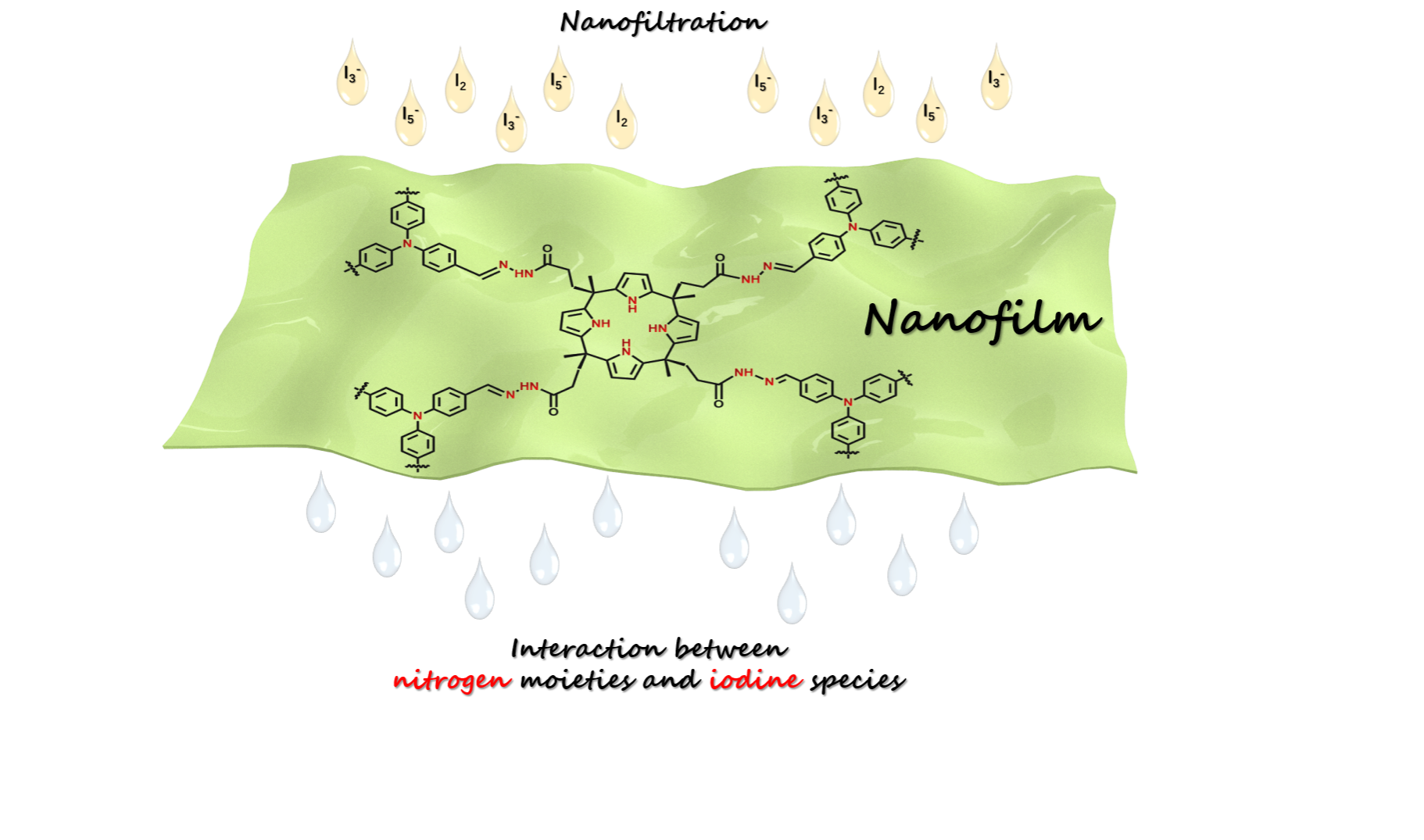
Nuclear energy has made great contributions to the progress of technology and the development of society. On the other hand, it produces massive radioactive wastes, which emerge as a serious challenge toward the environment and public health. Radioactive iodine isotopes (131I or 129I) generated by uranium fission are one of the numerous sources of radiation. The radioactive iodine (129I) must be disposed properly as it features with high toxicity, long half-life (1.57 × 107 years), strong radiation, and large mobility. Therefore, capture of iodine from vapor phase or solution becomes a pre-requirement. To date, a great majority of studies have concentrated on the capture of iodine vapor or iodine from organic solvent. Relatively few but equally important studies have focused on iodine adsorption from aqueous solutions. Practically, water contaminated by radioactive iodine has been occurred by mainly two sources that are the discharge of wastewater from nuclear power plant (e.g., Chernobyl and Fukushima), and hospitals. In addition, iodine is also used as a water disinfectant, especially in emergencies. Although safeguard procedures are seriously implemented prior to discharge, the residual iodine (e.g.,5-32 mg×L-1) is still harmful. Thus, development of techniques for removal of trace iodine from water is highly needed.
Adsorption method is prevalent for industrial 129I removal. The adsorbents reported include mainly silver-based solid adsorbents, activated carbons, metal-exchanged zeolite, clays, and others. Recently, new adsorbents with high surface areas and controllable porous structures, such as metal-organic frameworks (MOFs), covalent organic frameworks, porous aromatic frameworks, and other porous organic polymers, have been reported for iodine vapor capture. The adsorbent materials usable for capture of iodine from aqueous solution are not only limited, but also suffer from some shortcomings. For example, the nanomaterials-based adsorbents are easy to aggregate, which will decrease the accessibility of the binding sites. For porous materials-based adsorbents, the ratio of accessible binding sites are limited due to their unfavorable locations, the surface of internal pores. Therefore, membrane separation technology has emerged as another method for the capture of iodine from aqueous solution because of their merits of high decontamination factor, large volume reduction and low energy consumption. For instance, polymer/MOFs nanocomposite membrane was used for the quick removal of iodine from aqueous solutions.
It is to be noted, however, that most studies are mainly focused on the removal of iodine at high concentrations, and the important and challenging removal of iodine at trace level seems to be ignored. In this work, we attempt to employ nanofiltration to removal trace iodine for deep purification of water. Accordingly, a defect-free, self-standing calix[4]pyrrole-based nanofilm was designed and used for the deep purification of iodine contaminated water. The adsorption mechanism behind was explored.
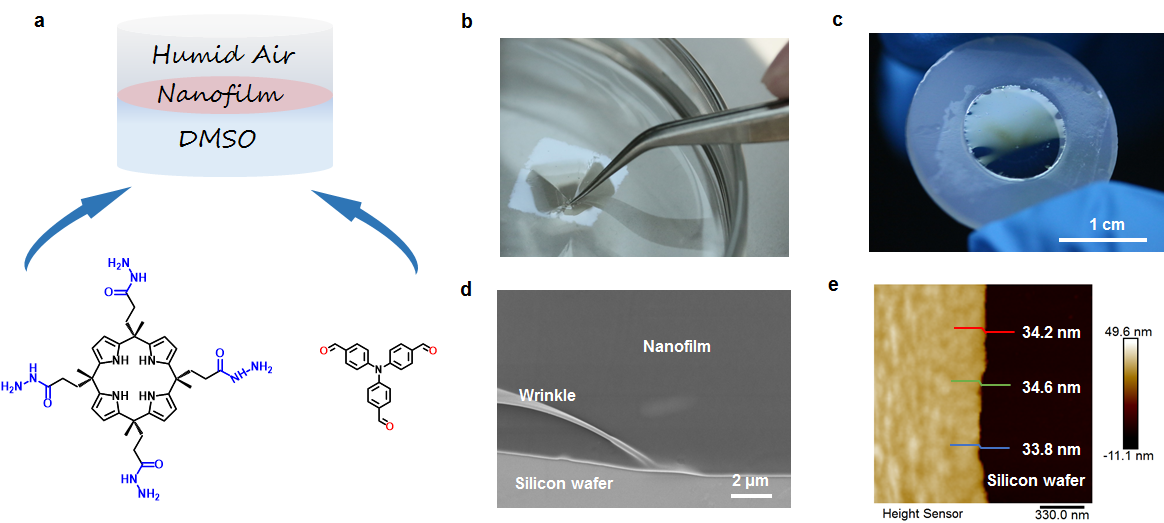
Figure 1. (a) Synthesis of CPTH-TFPA nanofilm. (b) Photograph of CPTH-TFPA nanofilm resistant to pointed tweezers. (c) Photograph of the nanofilm supported on a rubber ring. (d) SEM image of CPTH-TFPA nanofilm. (e) AFM image of CPTH-TFPA nanofilm on a silicon wafer.
Efficient removal of radioactive iodine from aqueous solution is largely dependent on the adsorbent materials employed. In this work, we report a calix[4]pyrrole-based nanofilm and its application for the rapid removal of iodine from water. The nanofilm was synthesized through a confined dynamic condensation of tetra hydrazide calix[4]pyrrole with 1,3,5-tri-(4-formylphenyl) aldehyde at the air/dimethyl sulfoxide (DMSO) interface. The thickness of the obtained nanofilm is ~35 nm, enabling fast mass transfer and high ratio of accessible binding sites for iodine. The pseudo-second-order rate constant of the nanofilm for iodine is ~0.061 g×g-1×min-1, three orders of magnitude higher than most reported adsorbent materials. Flow-through nanofiltration tests demonstrated that the nanofilm has an adsorption capacity of 1.48 g×g-1, high removal efficiency, and good reusability. Mechanism study revealed that the moieties of Schiff base, pyrrole and aromatic rings play a key role for binding iodine. We believe this work provides not only a new strategy for efficient removal of radioactive iodine from water, but also new ideas for designing efficient iodine adsorbents.
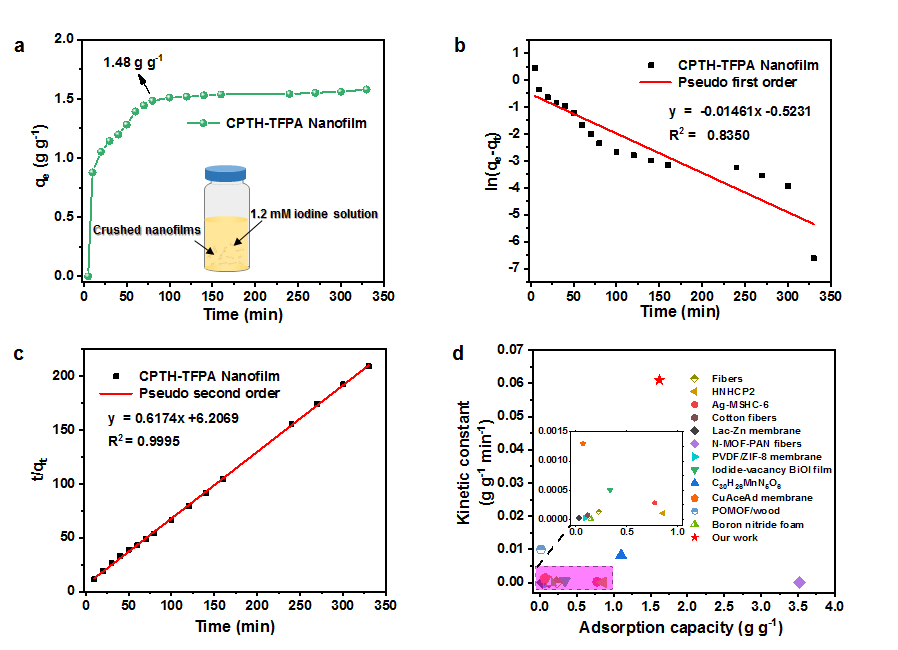
Figure 2. (a) Iodine adsorption kinetic of CPTH-TFPA nanofilm in I2 /KI aqueous solution with an initial iodine concentration of 1.2 mM. (b, c) Adsorption kinetic curves of CPTH-TFPA nanofilm. (d) Comparison of Iodine adsorption capacities and kinetic constant between reported membrane materials and our CPTH-TFPA nanofilm.
First Author: Wang Tingyi, master’s candidate, Shaanxi Normal University
Correspondence Authors: Prof. Fang Yu, Assoc. Prof. Liu Zhongshan, Shaanxi Normal University
Full Text Link: https://doi.org/10.1021/acs.langmuir.3c03961