
Yimin Bai, Jiman He, Yuting Gao, Miaomiao Zhang, Dexia Zhou, Yun Tang, Jing Liu, Hongtao Bian*, and Yu Fang. J. Phys. Chem. B, 2024, 128, 2447-2456.
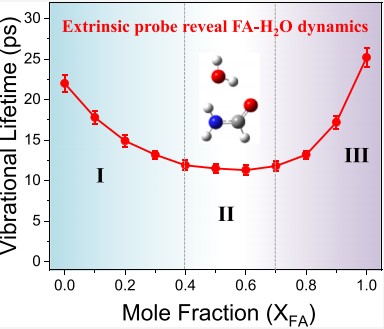
Formamide (FA) stands as one of the smallest molecules featuring amide bonds, enabling the formation of C=O···H and NH···O hydrogen bonds. The three-dimensional hydrogen bonds within liquid FA create a solvent-like state reminiscent of water’s properties. Their mutual miscibility fosters microhomogeneous networks, rendering FA an invaluable model for studying essential biophysical processes driven by hydrogen bonding. This study is focused on the dynamics of FA−water mixtures using linear and femtosecond infrared spectroscopies. By using the intrinsic OD stretch and extrinsic probe SCN−, the local vibrational behaviors and hydrogen bonding dynamics across various FA−water compositions were systematically investigated.
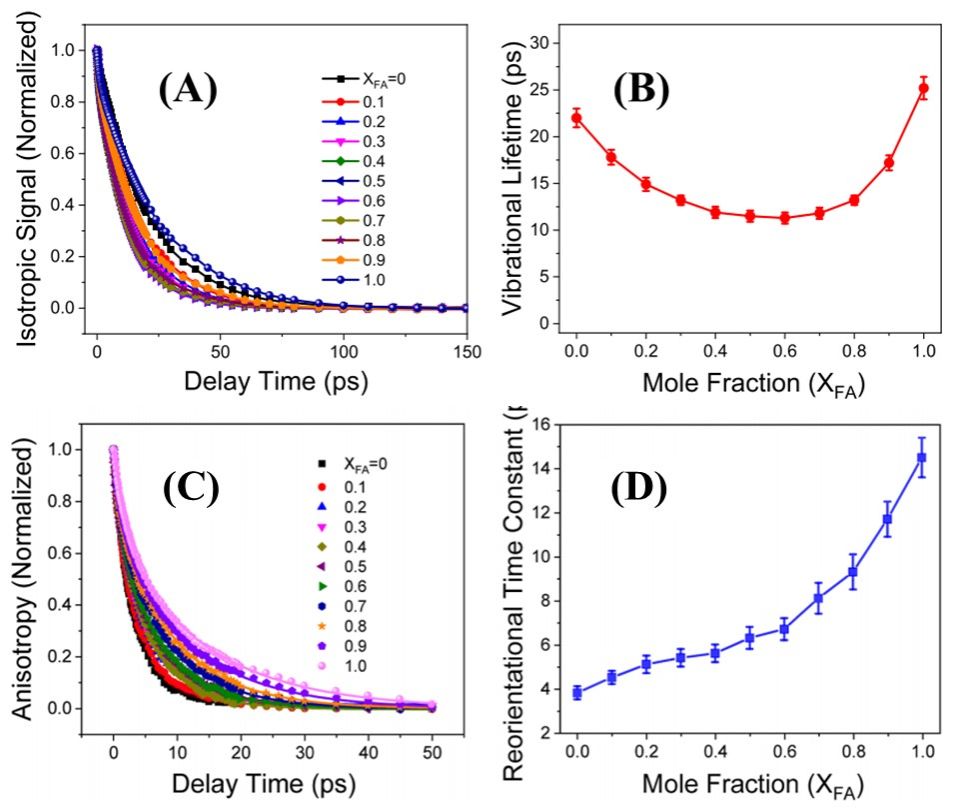
Figure 1. (A) Normalized vibrational population relaxation curves for the antisymmetric stretch of SCN− in FA aqueous solutions with different concentrations. (B) Concentration-dependent vibrational lifetime (slow component) of SCN− stretch (C) Anisotropy decay of SCN− probe in the FA aqueous solutions. All the anisotropy decay curves are normalized to 1 at the delay time of zero. The solid lines are the fitting data using biexponential decay function. (D) Concentration-dependent rotational time constants of SCN− stretch.
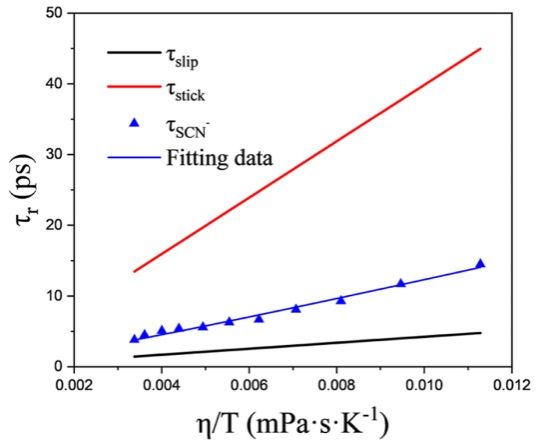
Figure 2. Plot of 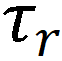 versus
versus 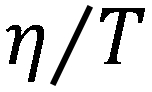 for SCN− in different concentrations of FA−water mixture solutions and in the two cases of the hydrodynamic stick and slip boundary conditions represented by the red and black lines, respectively. The blue line represents the fitting result.
for SCN− in different concentrations of FA−water mixture solutions and in the two cases of the hydrodynamic stick and slip boundary conditions represented by the red and black lines, respectively. The blue line represents the fitting result.
In this work, the vibrational relaxation of OD stretch revealed a negligible impact of FA addition on the vibrational lifetime of water molecules, underscoring the mixture’s water-like behavior. However, the reorientational dynamics of OD stretch slowed with increasing FA mole fraction, plateauing beyond XFA > 0.5. This suggests a correlation between OD’s reorientational time and the strength of the hydrogen bond network, likely tied to the solution’s changing dielectric constant. The vibrational relaxation dynamics of SCN− was strongly correlated with XFA, highlighting a competition between water and FA molecules in solvating SCN−. There’s also a linear relationship between rising viscosity and the prolonged correlation time of SCN−’s slow dynamics indicates that the solution’s macroscopic viscosity is dictated by the extended structures formed between FA and water molecules. The relation between the reorientation dynamics of the SCN− and the macroscopic viscosity in aqueous FA−water mixture solutions was analyzed by using the Stokes−Einstein−Debye equations. The direct viscosity-diffusion coupling is observed, which can be attributed to the homogeneous dynamics feature in FA−water mixture solutions. This study offers fundamental insights into the dynamics of FA−water mixture solutions, highlighting their molecular-level understanding of the homogeneity within the binary mixture.
First Author: Bai Yimin, Master’s candidate, Shaanxi Normal University
Correspondence Authors: Prof. Bian Hongtao, Shaanxi Normal University
Full Text Link: https://doi.org/10.1021/acs.jpcb.3c07850