
Yushu Li, Huizhen Han, Xiuhang Wang, Yu Sun, Yulian Zhao, Shiyi Tao, Aoxing Duan, Yi Ma*, Xin Bo*, and Zenglin Wang*. Chem. Commun., 2024, 60, 4052-4055.
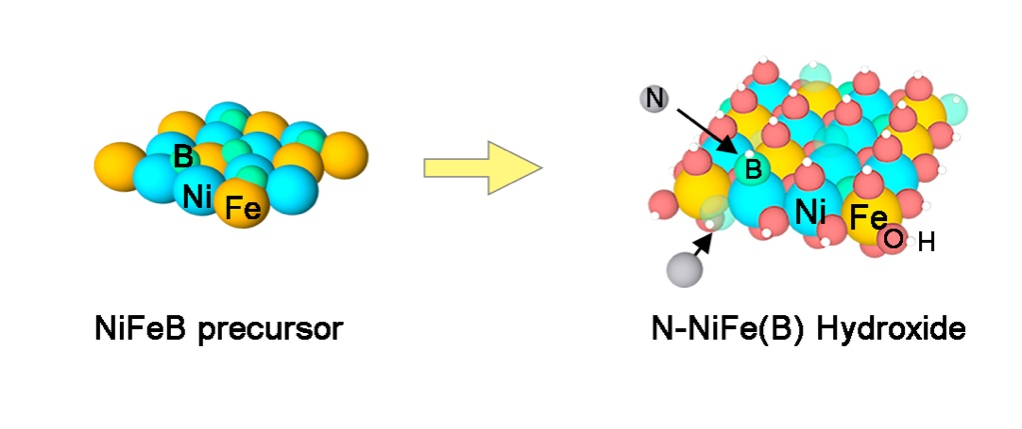
N-doped (oxy)hydroxide systems can effectively enhance the electrochemical water oxidation catalytic activity. However, conventional N doping requires extreme conditions such as high temperature, high pressure, and specific atmospheres, which are not conducive to the large-scale preparation of catalytic materials. Our team utilized the semi-metallic nature and large atomic radius of the non-metallic boron element to prepare NiFeB interstitial solid-solution alloy precursors by electroless plating; subsequently, the precursor was aged into (oxy)hydroxide under the mild conditions (merely 120 °C), during which boron atoms were substituted with ammonia in the gas phase, resulting in boron-induced N-doped NiFe(B) (oxy)hydroxide monolithic electrode. The catalytic activity of the active electrode for oxygen evolution in alkaline media is significantly enhanced with an onset overpotential of 225 mV. At the same time, the influence of factors such as electronic structure, electrochemical surface area, and hydrophilicity of the material on the catalytic activity were systematically investigated. In addition, this method can be further extended to the sulfur doping. This study provides technical support for the large-scale preparation of catalytic electrodes for electrochemical water oxidation, promoting China's Carbon Peaking and Carbon Neutrality process.
First Authors: Yushu Li, Huizhen Han, master’s candidates, Shaanxi Normal University
Correspondence Authors: Prof. Wang Zenglin, A/Prof. Bo Xin, A/Prof. Ma Yi, Shaanxi Normal University
Full Text Link: https://pubs.rsc.org/en/content/articlelanding/2024/cc/d3cc05190k
 Latest Updates
Latest Updates






