
Yangtao Shao, Rongrong Huang*, Guanyu Jiang, Qiyuan Shi, Hexi Wei, Gang Wang, Weijie Chi, Haonan Peng*, Xiaogang Liu*, and Yu Fang*. Chem. Mater., 2024, 36, 3223-3232.
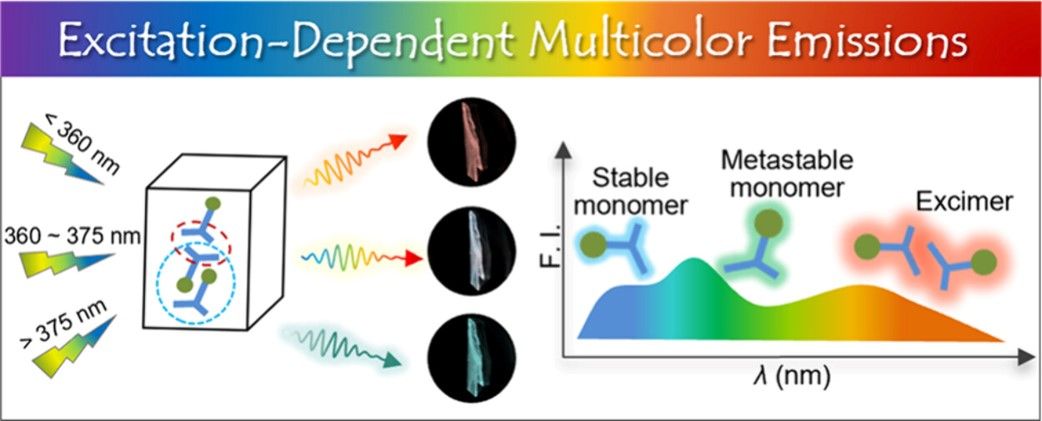
Multicolor luminescent materials with tunable properties hold great promise for a wide range of applications in materials science. Unfortunately, the conventional approach to achieving multicolor emissions by blending multiple types of fluorophores is hindered by limitations, notably, spectral instability, aggregation-caused quenching, and energy transfer. The pursuit of multicolor emissions from a single type of fluorophore in the solid state has, until now, remained a formidable challenge.
The key to creating single-fluorophore-based multicolor emissive materials lies in the ability to generate multiple emissive centers (preferably more than two). This can be achieved by carefully controlling ground-state and/or harnessing intra-/intermolecular excited-state structural transformations, and challenging Kasha’s rule (as illustrated in Figure 1).
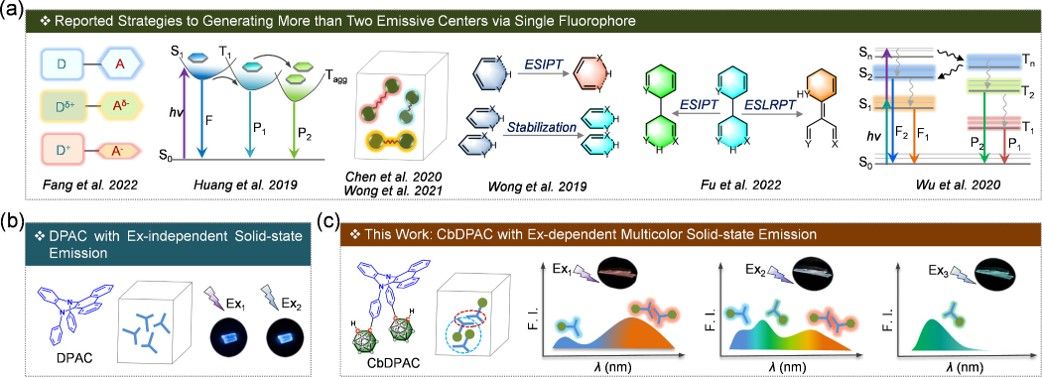
Figure 1. (a) Schematic Illustrations for the Reported Strategies for Generating More than Two Emissive Centers via Single Fluorophores. (b) Fluorescent Properties of DPAC in the Solid State, and the Cartoon Illustration Showing the Packing Form of the DPAC Crystal. (c) Schematic Illustration for the Excitation-Dependent Multicolor Emission of the CbDPAC Crystal, and the Cartoon Illustration Showing the Packing Form of the CbDPAC Crystal.
In this study, we have introduced N,N′-diphenyl dihydrodibenzo[a,c]-phenazines (DPAC), augmented with two o-carboranyl units, to create a novel fluorophore CbDPAC. The CbDPAC crystal exhibits three distinct emission bands peaking at 405, 470, and 620 nm, respectively, arising from a rich intermolecular interaction network that generates novel emission centers, such as conformational isomers and excimers. This work inspires the rational molecular engineering of smart fluorophores with tailorable properties and inaugurates diverse possibilities for stimuli-responsive luminescent technologies.
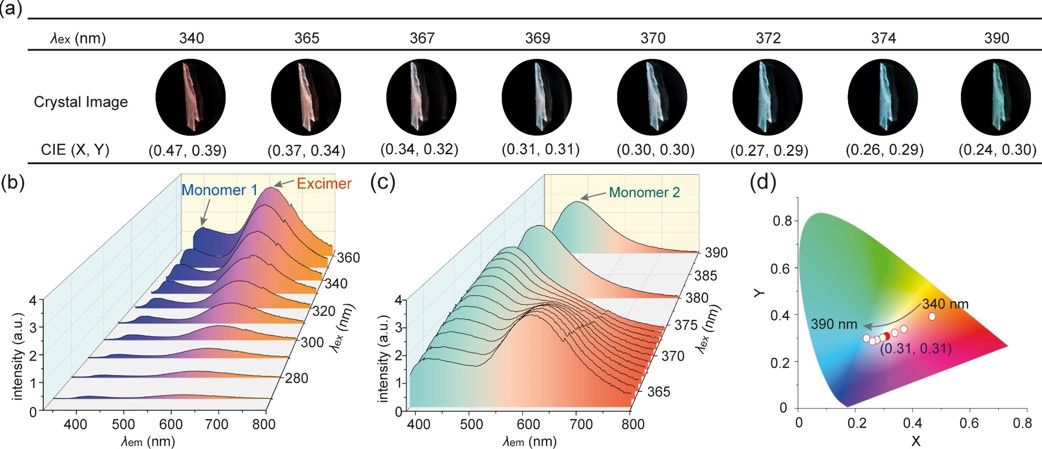
Figure 2. (a) Fluorescence images of the CbDPAC crystal under illumination at various wavelengths and the corresponding Commission International de l’Eclairage (CIE) values. (b, c) Fluorescence spectra of the CbDPAC crystal recorded at various excitation wavelengths. (d) CIE coordinate diagram of crystal CbDPAC under illumination at various wavelengths. The red dot represents the CIE value under the excitation wavelength of 369 nm. Notes: monomer 1 and monomer 2 refer to stable and metastable conformational isomers, respectively.
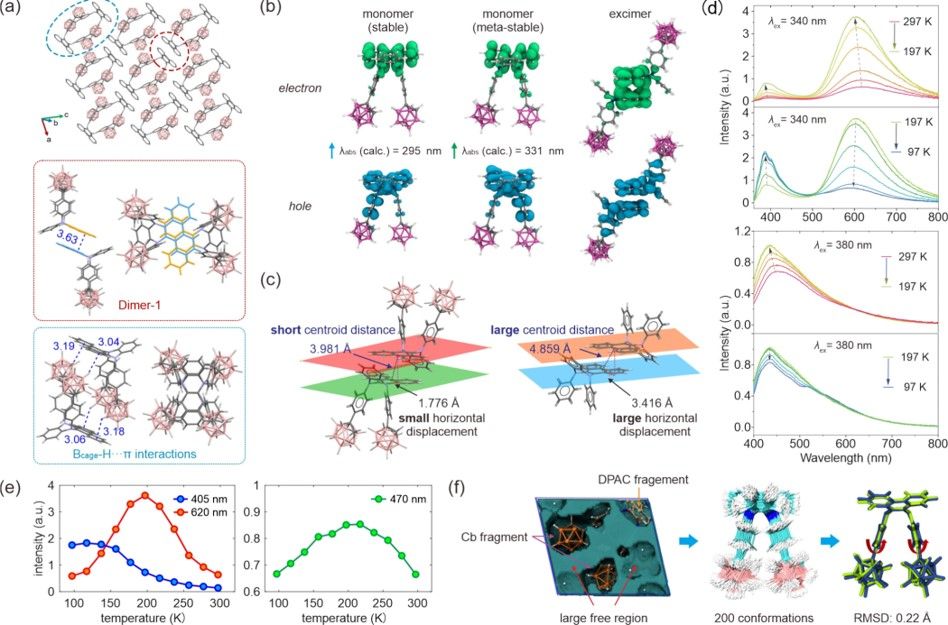
Figure 3. (a) Molecular packing of CbDPAC (top), where hydrogen atoms are omitted for clarity, and two types of intermolecular interactions are highlighted in red and blue circles. The picture in the red circle shows the intermolecular π···π interactions in dimer-1 (middle), and the picture in the blue circle shows intermolecular Bcage-H···π interactions (bottom). (b) Electron and hole distributions of the stable monomer (left), metastable monomer (middle), and excimer (right). The calculated UV−vis absorption wavelengths of the stable and metastable monomers are shown in the inset. Dimer 1, optimized in its ground state, acts as a proxy for the “excimer” due to the prohibitive costs associated with modeling its excited state. (c) Comparison of the inter-DPAC centroid distance and horizontal displacement in the CbDPAC crystal and the reference DPAC crystal. (d) Temperature-dependent fluorescence spectra of the CbDPAC crystal excited at 340 and 380 nm. (e) Fluorescence intensity of the three emission peaks (405, 620, and 470 nm) as a function of temperature. (f) Free volume (left) in the CbDPAC crystal, the overlapping structures of the 200 CbDPAC conformers (middle), the overlapping of the stable (in yellow) and metastable (in blue) monomer conformations (right), and the corresponding root-mean-square deviation (RMSD) of the two conformations.
First Author: Shao Yangtao, doctoral candidate, Shaanxi Normal University
Correspondence Authors: Prof. Fang Yu and Prof. Peng Haonan, Shaanxi Normal University; Prof. Liu Xiaogang and Dr. Huang Rongrong, Singapore University of Technology and Design
Full Text Link: https://doi.org/10.1021/acs.chemmater.3c02993