
Vilma Lovrinčević, Dexin Zheng, Mélyne Baudin-Marie, Mia Marić, Lidija Uzelac, Irena Škorić, Jiani Ma*, and Dragana Vuk*. J. Photochem. Photobiol. A Chem. 2024, 454, 115715.
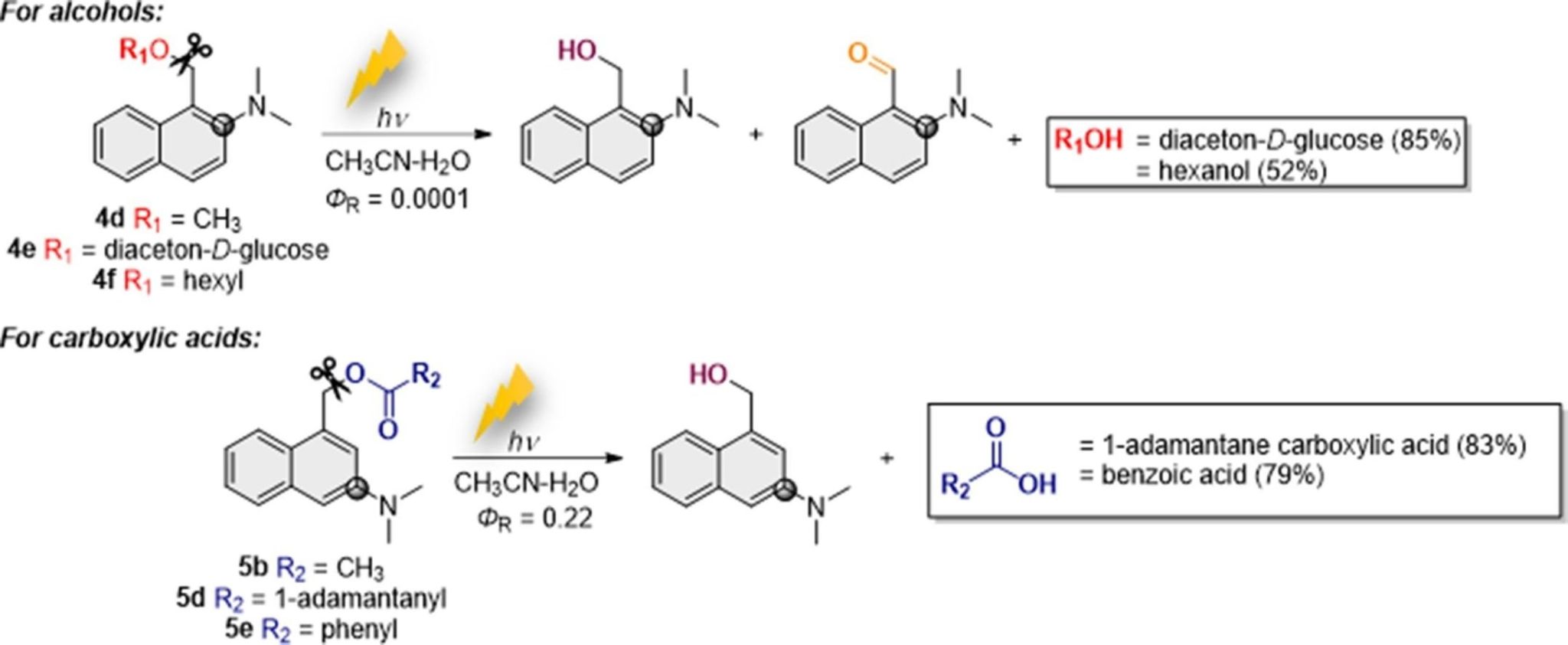
The photolabile protective group (PPG) can mask the chemical and biological activity of the protected group. By selecting the appropriate wavelength of light for irradiation, the target molecule can be accurately released and its performance can be restored. Wang et al. developed a specific line of photocages that base their photoreactivity on the Zimmerman’s meta-effect, and they demonstrated the use of m-hydroxymethylaniline photocages for alcohols. Furthermore, Wang et al. and we have shown that o-hydroxymethylaniline derivatives can also be used as photocages for alcohols and carboxylic acids. The photoreactivity of these o-aniline derivatives can be modified towards the release of acids or alcohols by introducing a methyl group in the reactive center. However, aniline photocages absorb light at λ < 350 nm, which reduces their applicability in biological systems. To address this problem we extended the chromophoric system and developed N,N-dimetyhlaminonaphthalene photocages, which absorb at near-visible region and can be used together with anilines for chromo-orthogonal deprotection. Further investigation led to the development of substituted aminonaphthalenes, which react more efficiently in releasing carboxylates. For this reason, 1,3-substituted N,N-dimetyhlaminonaphthalene photoprotective groups (denoted as 5b) were selected as the representative system to study the photodeprotect mechanism (Scheme 1).
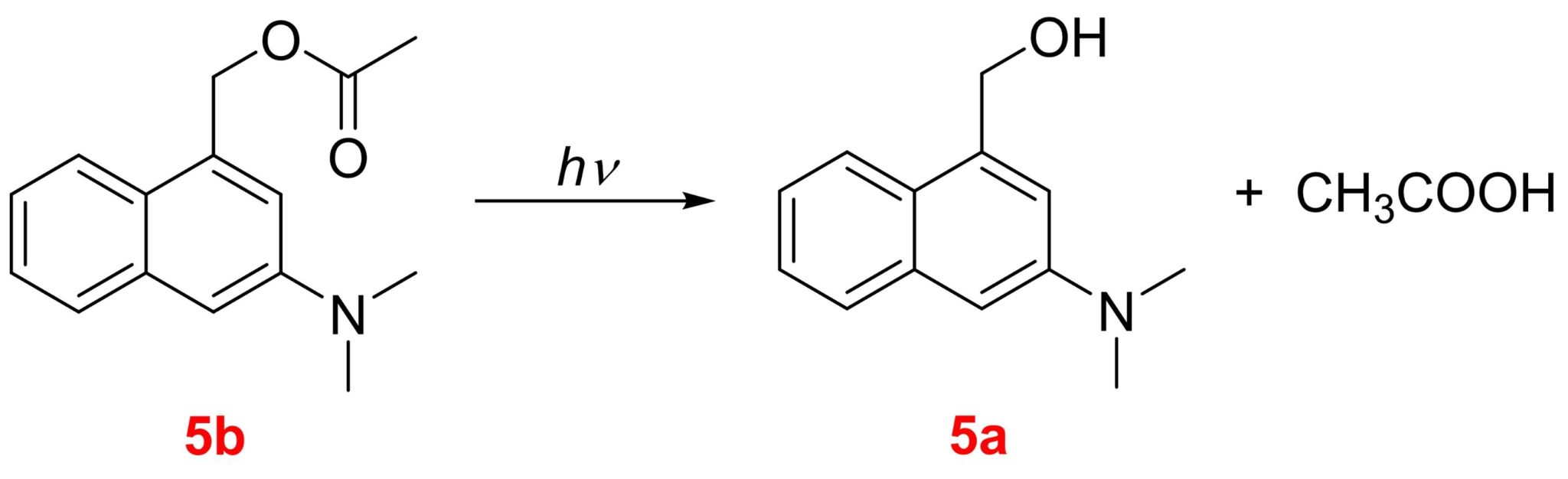
Scheme 1. Photorelease reaction of 5b
The femtosecond transient absorption and nanosecond transient absorption coupling with density functional theory were employed to thoroughly investigate the photorelease mechanism. The photodeprotection mechanism of 5b is depicted (Scheme 2). The results show that the photoelimination of carboxylates takes place directly in the singlet excited state by a homolytic cleavage producing a radical pair. The subsequent electron transfer gives rise to aminonaphthalene carbocation and the carboxylate.
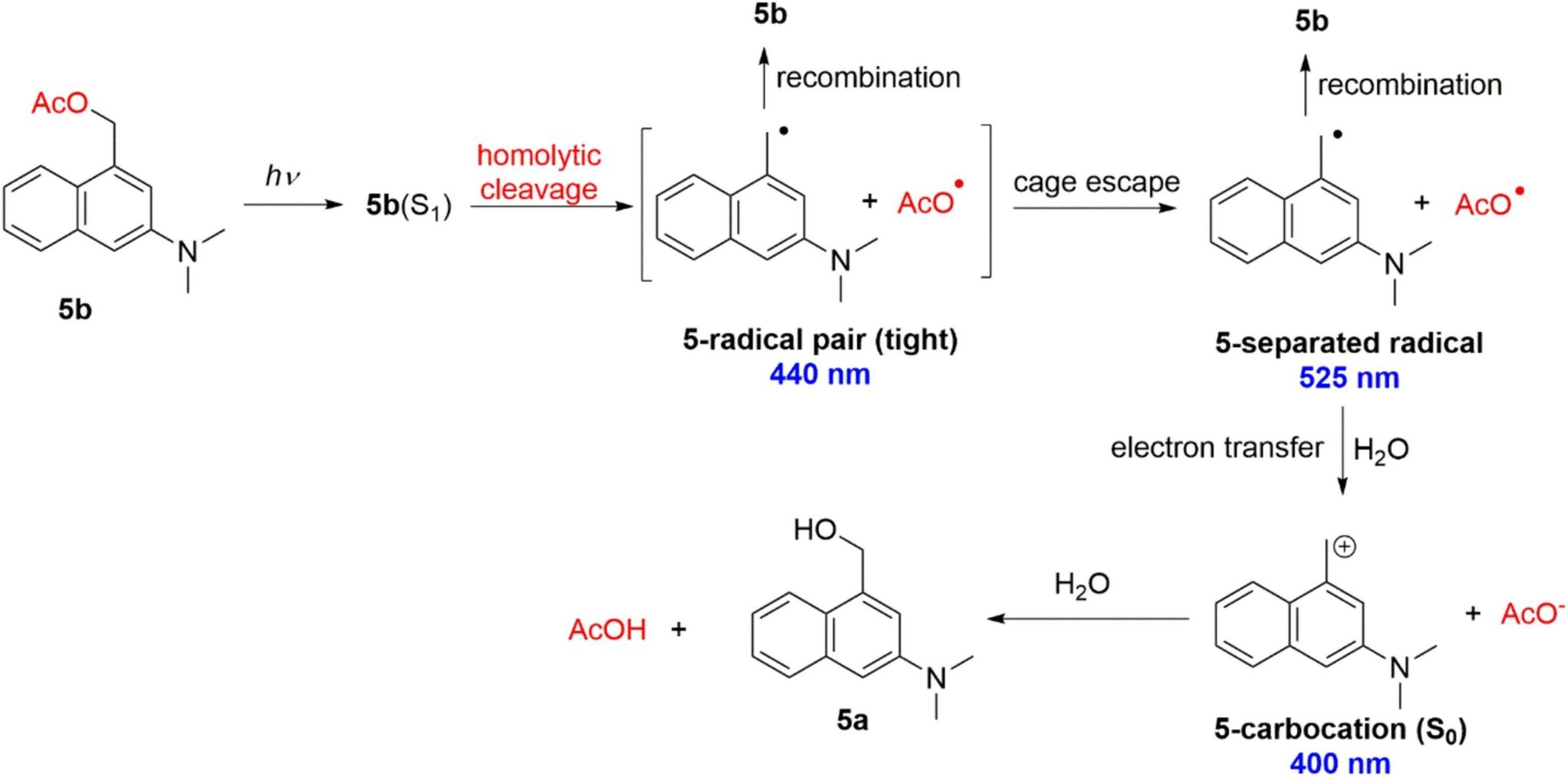
Scheme 2. Proposed photodeprotection mechanisms of 5b
First Author: Vilma Lovrinčević, graduate student, University of Zagreb
Correspondence Authors: Prof. Dragana Vuk, University of Zagreb; Prof. Ma Jiani, Shaanxi Normal University
Full Text Link: https://www.sciencedirect.com/science/article/pii/S1010603024002594