
Yan Guo, Hongtao Bian, Le Yu, Jiani Ma*, and Yu Fang. Chin. Chem. Lett., 2024, 109971. DOI: 10.1016/j.cclet.2024.109971.
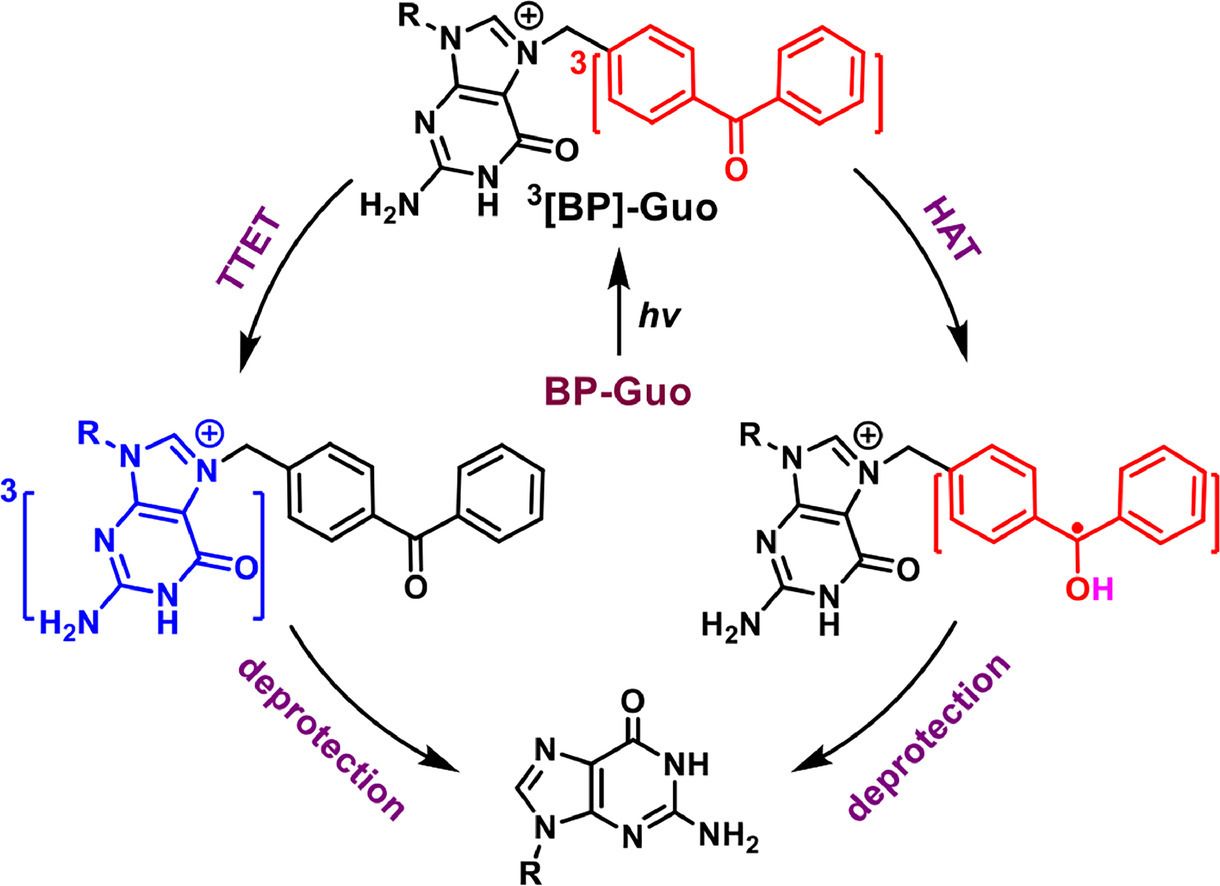
Establishing the precise conditional control of biological function comparable to that of the natural system is an important tool for elucidating the mechanisms of cellular processes. One of the most excellent strategies is photoregulation by introducing photolabile protecting group (PPG) to small molecules, proteins, and nucleic acids. After light irradiation at a suitable wavelength, the protecting group is irreversibly cleaved whereby the masked native functionality and biological activity of the protected compound can be restored. Unlike the typical protecting strategies of nucleobases, in which PPG replaces a hydrogen atom with modifications and does not change the charge distribution of the molecule, Rentmeister developed a novel strategy to protect nucleic acids by introducing a positive charge on nucleobase. And this strategy is extended to more complex biomolecules dinucleotide and RNA, achieving to spatiotemporally control biologically relevant functions. In order to improve the efficiency of photodeprotection reaction and to provide design principles for high-performance PPGs, Benzophenone protected guanosine at N7 position (denoted as BP-Guo) was selected as the representative system to study the photodeprotect mechanism (Figure 1).
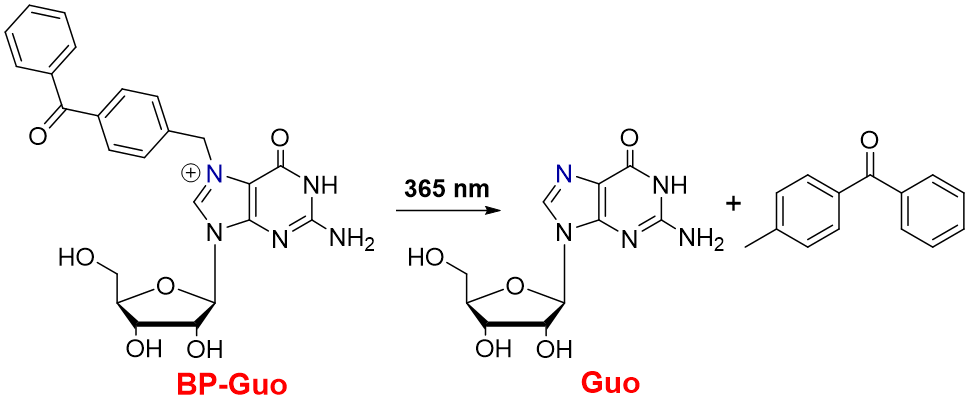
Scheme 1. Photorelease reaction of Guo from BP-Guo
The femtosecond transient absorption, nanosecond transient absorption, and nanosecond transient resonance Raman spectroscopy coupling with density functional theory were employed to thoroughly investigate the photorelease mechanism. The photodeprotection mechanism of BP-Guo is depicted (Figure 2). After generation of 3[BP]-Guo, two competing reaction routes occurred to trigger the photodeprotection. One route is the TTET to yield BP-3[Guo], which breaks C-N bond to release the Guo along with BP cation that finally trapped by solvent to give the final product. The other is the HAT with formation of ketyl-Guo, followed by releasing Guo and the BP radical cation. The later undergoes electron transfer and further tautomerizes to 4-methylBP. The present results provide an in-depth insight into the inactivation pathway for BP protected nucleic acids after light irradiation. In particular, the photosensitized TTET pathway from BP leads to the generation of nucleic acid in their triplet states, which, in addition to potentially initiating photodeprotection, may also trigger well-known cyclization reactions that brings about DNA mutagenic adducts, commonly referred to as triple photodamage. The latter needs further investigation and should be avoided in developing improved strategies for photoprotecting nucleic acids.
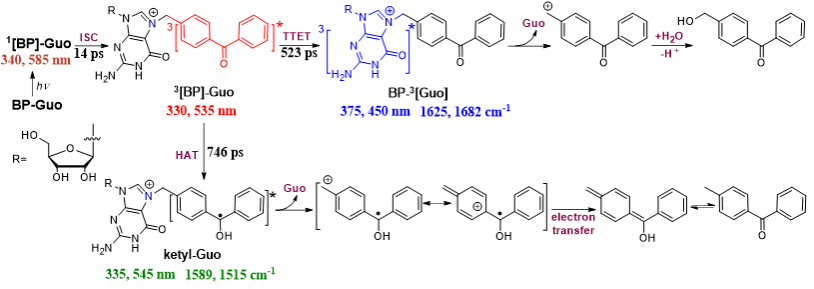
Scheme 2. Proposed photodeprotection mechanisms of BP-Guo in glycerol-ACN-H2O
First Author: Dr. Guo Yan, Shaanxi Normal University
Correspondence Author: Prof. Ma Jiani, Shaanxi Normal University
Full Text Link: https://www.sciencedirect.com/science/article/pii/S100184172400490X