
Shuang Xu, Tianhe Yang, Shu-Lin Zhang, Yifan Su, Yang Cheng, David Lee Phillips, Le Yu*, Jiani Ma*, and Yu Fang. J. Phys. Chem. Lett. 2025 DOI: https://doi.org/10.1021/acs.jpclett.5c00720
Photochemistry is considered one of the most efficient and reproducible techniques in organic synthesis. Recently, List and co-workers reported an efficient UV light triggered photochemical synthesis of spiro[2,4]heptadiene from fulvenes with different substituents ( Angew. Chem. Int. Ed. 2023, 62, e202303119); however, the mechanistic details remain unclear, and the intermediates have not been characterized. To facilitate the applications of this novel photochemical reaction, we theoretically designed a series of fulvene derivatives with different parent molecular skeletons for analyzing the substitution effects, and two of the representative fulvenes were synthesized for investigating the reaction mechanisms by employing time-resolved transient absorption spectroscopy (TA) experiments. It has been found that instead of density functional theory, the second-order n-electron valence state perturbation theory is necessary to acquire reliable theoretical characterization of the fulvenes examined. Our designed fulvenes were found to undergo the photorearrangement cyclopropanation reaction on the basis of photoproduct analysis. The intermediate species involved in the intramolecular hydrogen atom transfer and cyclization processes within the photorearrangement reaction were characterized by TA spectroscopy, and the full reaction pathways were proposed. Our work not only reveals the detailed mechanism of this photorearrangement reaction but also demonstrates the significance of appropriate theoretical methods for rational molecular design.
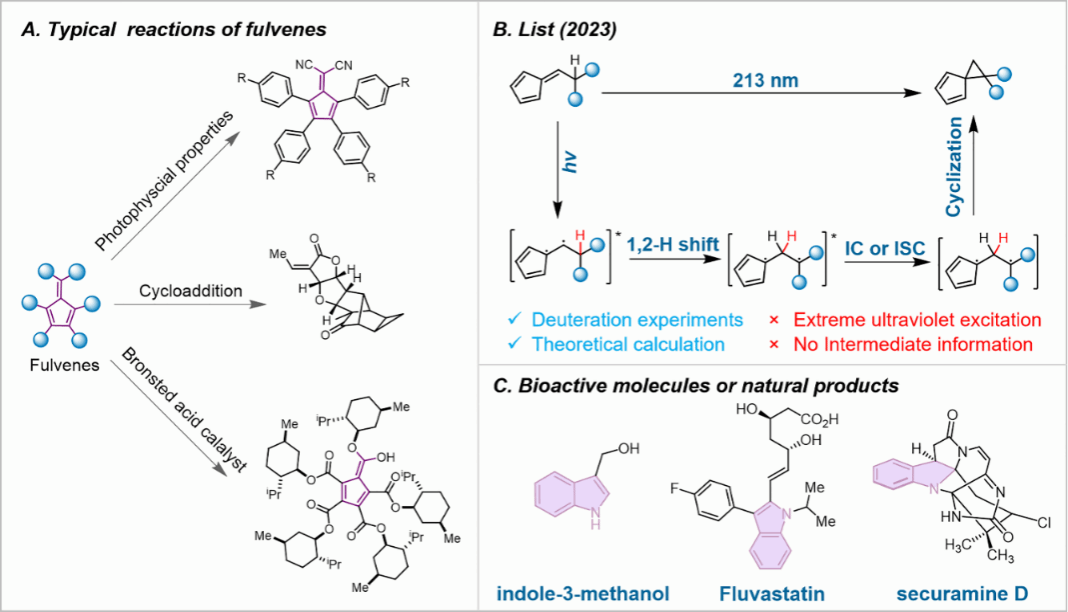
Figure 1. (A) Examples of typical reactions of fulvenes, (B) proposed photo-rearrangement mechanism for the fulvenes by List, (C) heterocyclic-containing drug and natural product molecules.
After rational design and screening of 34 candidates using theoretical computations, two new fulvene molecules were successfully synthesized. The molecular design increases greatly, expanding the scope of spiro[2,4]heptadiene synthesis upon irradiation of ∼300 nm. Moreover, the theoretical analysis reveals that for derivatives with −MOP and −THP substituents, the inaccuracy of the DFT/TDDFT screening for fulvenes with −MOP and −THP substitutions confirmed the necessity of utilizing NEVPT2/CASSCF computations in the rational design of other photochemical reactions with similar molecule backbones.
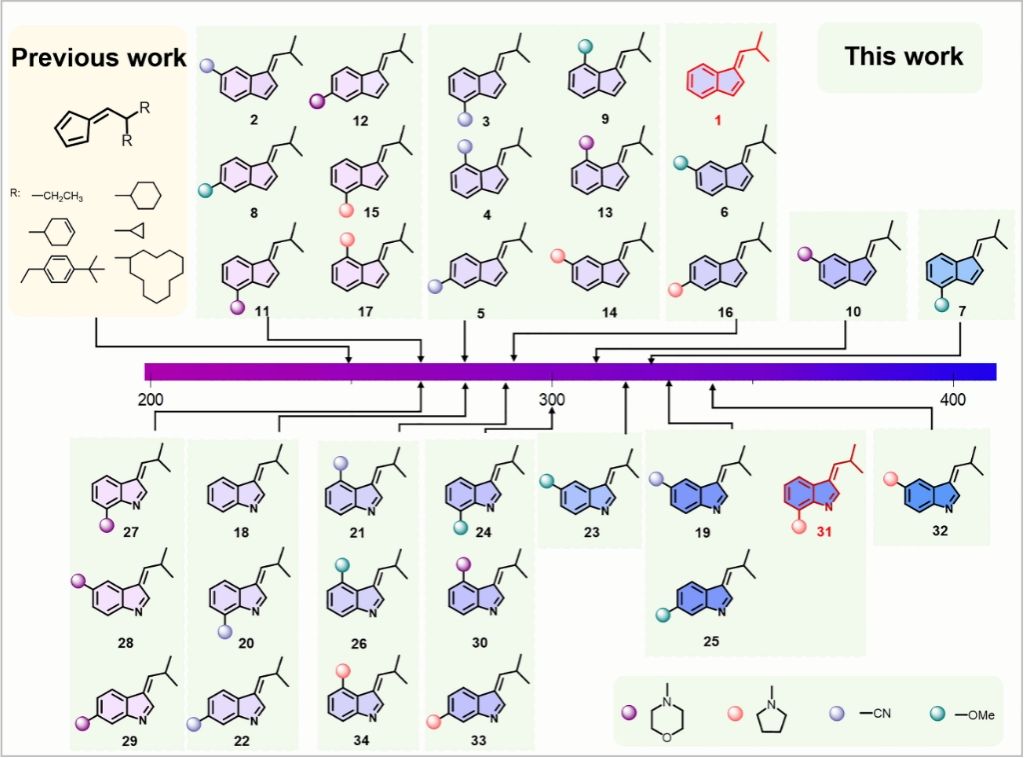
Figure 2. The structures of the screened fulvene molecules with electronic absorbance simulated by NEVPT2(8,8)/def2-TZVP//CASSCF(6,6)/6-31G(d).
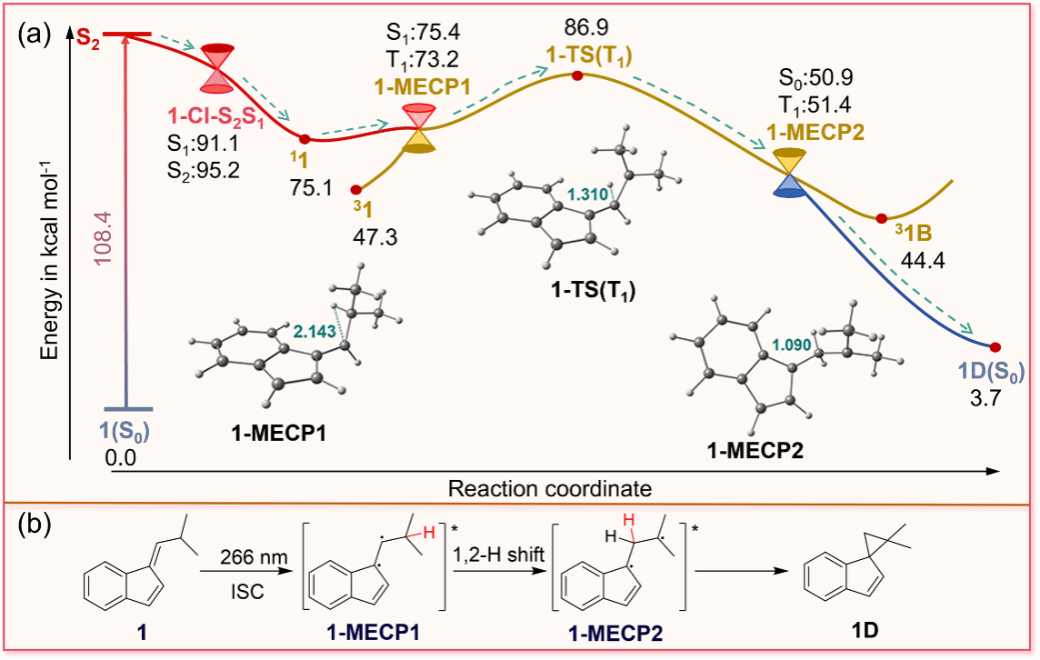
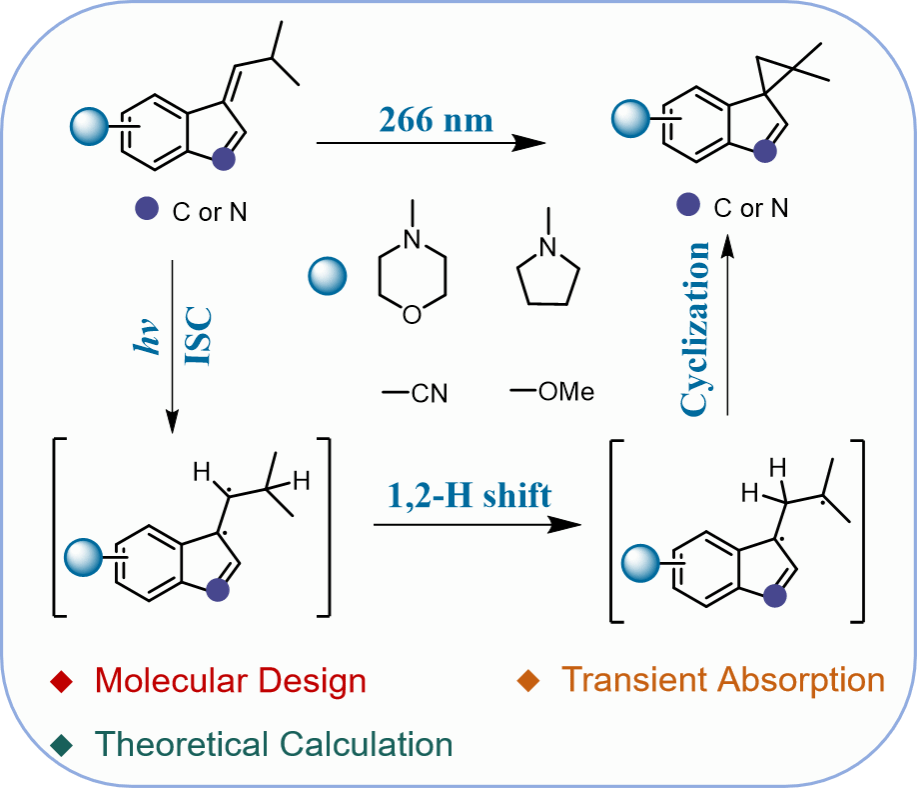
Figure 3. (a) Potential energy profile of 1 computed at NEVPT2(8,8)/def2-TZVP//CASSCF(6,6)/6-31G(d) level of theory (the energy is kcal mol-1 and ground state energy of 1(S0) was used as zero energy for estimating relative energies, the geometry of the transition state 1-TS(T1) was optimized by TDDFT) and ISC channels at 1-MECP1, (b) the proposed photochemical reaction mechanism of 1.
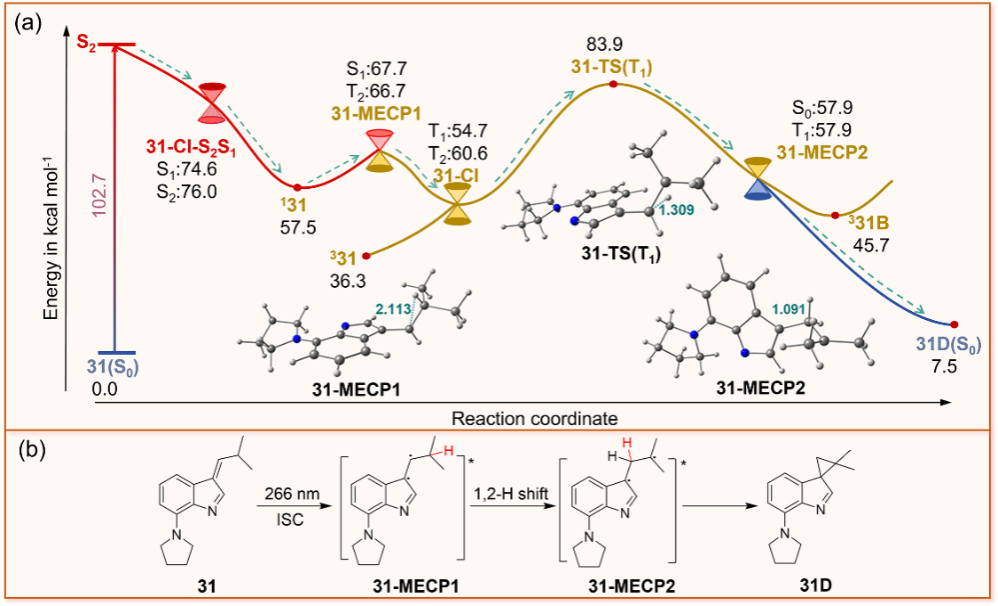
Figure 4. (a) Potential energy profile of 31 computed at NEVPT2(8,8)/def2-TZVP//CASSCF(6,6)/6-31G(d) level of theory (the energy is kcal mol-1 and ground state energy of 31(S0) was used as zero energy for estimating relative energies, the geometry of the transition state 31-TS(T1) was optimized by TDDFT) and ISC channels at 31-MECP1, (b) the proposed photochemical reaction mechanism of 31.
With the help of state-of-the-art ultrafast spectroscopies and high-level ab initio computations, the detailed photorearrangement mechanism was determined for both molecules. 1 and 31 follow a similar reaction mechanism to generate the photorearrangement product. The triplet state of the substrate and the diradical species involved in the intramolecular HAT process were directly detected experimentally. The calculation results show that after the molecule is excited to the singlet excited state, a HAT process occurs around the ISC point, followed by a cyclization to generate the product. On the other hand, it was found that the reaction rate of 31 was much slower, compared to that of 1. First, the energy barrier of the HAT process for 31 is 13.7 kcal mol–1 higher than that for 1, and the reaction rate constant ratio of 1 to 31 is calculated to be k1/k31 = 74.4 using the Arrhenius formula. Second, the ISC rate constant for 31 (3.03 × 105 s–1) is smaller than that for 1 (1.73 × 108 s–1), further demonstrating the slower reaction rate of 31.
First Author: Xu Shuang, master’s student, Shaanxi Normal University
Correspondence Authors: Prof. Ma Jiani, Shaanxi Normal University; A/Prof. Yu Le, Northwest University
Full Text Link: https://doi.org/10.1021/acs.jpclett.5c00720